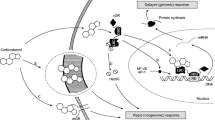
Abstract
Inhaled corticosteroids (ICS) are the most potent of all the available inhaled treatments, and are effective medications for long-term control of asthma. However, their use in children is limited by the risk of systemic adverse effects. Although results reported in the literature on the adverse effects of ICS are conflicting and often restricted to a small number of cases with a limited follow-up, most of them show an early decrease in growth velocity without significant influence on final adult height. Partial adrenal suppression has also been demonstrated in children treated with ICS for more than 2 months.
Only children with mild persistent, moderate, or severe asthma not controlled by non-corticosteroid drugs should be treated with ICS for long periods. The dose of ICS must be individually adjusted to minimize the possible adverse effects on growth, and all children with asthma receiving long-term treatment with ICS must be regularly evaluated for growth impairment, which may necessitate dose reduction or drug replacement.







Similar content being viewed by others
References
National Asthma Educational Program. Guidelines for the diagnosis and management of asthma. Bethesda (MD): US Department of Health and Human Services, 1998
Centers for Disease Control and Prevention. Surveillance for asthma: United States, 1960–1995. MMWR CDC Surveill Summ 1998; 47Suppl. 1: 1–27
Scott TW. Epidemiology and heterogeneity of asthma. Ann Allergy Asthma Immunol 2001; 87Suppl. 1: 5–8
National Institutes of Health. Global strategy for asthma management and prevention. Bethesda (MD): National Institutes of Health, 1995 Jan. NHLBI/WHO workshop report no. 95-3659
National Education and Prevention Program. Guidelines for the diagnosis and management of asthma. Washington, DC: National Heart, Lung, and Blood Institute, Expert Panel Report 2, 1997 Jul. NIH Publication No. 97-4051
Van Essen-Zandvliet EE, Hughes MD, Waalkens HJ, et al. Effects of 22 months of treatment with inhaled steroids and/or beta-2-agonist on lung function, airway responsiveness, and symptoms in children with asthma. Am Rev Respir Dis 1992; 146: 547–54
Hardman JG, Limbird LE, editors. Goodman and Gilman: the pharmacological basis of therapeutics. 8th ed. New York: Pergamon Press, 1990
Johnson M. Pharmacodynamic and pharmacokinetics of inhaled glucocorticosteroids. J Allergy Clin Immunol 1996; 97: 169–76
Skoner DP. Balancing safety and efficacy in pediatric asthma management. Pediatrics 2002; 109: 381–92
Allen DB. Influence of inhaled steroids on growth: a paediatric endocrinologist’s perspective. Acta Paediatr 1998; 87: 123–9
Lipworth BJ, Jackson CM. Safety of inhaled and intranasal corticosteroids: lessons for the new millennium. Drug Saf 2000; 23: 11–33
Colice GL. Comparing inhaled corticosteroids. Respir Care 2000; 45(7): 846–53
Mager DE, Jusko WJ. Quantitative structure-pharmacokinetic/pharmacodynamic relationships of corticosteroids in man. J Pharm Sci 2002; 91(11): 2441–51
English AF, Neate MS, Quint DJ, et al. Biological activities of some corticosteroids used in asthma [abstract]. Am J Respir Crit Care Med 1994; 149: A212
Silverstein MD, Yunginger JW, Reed CE, et al. Attained adult height after childhood asthma: effect of glucocorticoid therapy. J Allergy Clin Immunol 1997; 99: 466–74
Anhoj J, Bisgaard AM, Bisgaard H. Systemic activity of inhaled steroids in 1- to 3-year-old children with asthma. Pediatrics 2002; 109(3): E40
The transition to CFC-free inhalers: what pharmacists should know. Pharm J 1998; 261: 316–7
Allen DB. Inhaled corticosteroid therapy for asthma in preschool children: growth issues. Pediatrics 2002; 109: 373–80
Tinkelman DG, Reed CE, Nelson HS, et al. Aerosol beclomethasone dipropionate compared with theophylline as primary treatment of chronic mild to moderately severe asthma in children. Pediatrics 1993; 92: 64–77
Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000; 343: 1064–9
Cohen MB, Abram LE. Growth pattern of allergic children. J Allergy 1948; 19: 165
Ninan TK, Russell G. Asthma, inhaled corticosteroids treatment and growth. Arch Dis Child 1992; 67: 703–5
Harter JG, Reddy JR, Thorn GW. Studies on an intermittent corticosteroids dosage regimen. N Engl J Med 1963; 269: 591–6
Byron MA, Jackson J, Ansell BM. Effect of different corticosteroid regimens on hypothalamic-pituitary-adrenal axis and growth in juvenile chronic arthritis. J R Soc Med 1983; 76: 452–7
Reinberg A, Gervais P, Chaussade M, et al. Circadian changes in effectiveness of corticosteroids in 8 patients with allergic asthma. J Allergy Clin Immunol 1983; 71: 425–33
Wolthers OD, Pedersen S. Knemometric assessment of systemic activity of once daily intranasal dry-powder budesonide in children. Allergy 1994; 49: 96–9
Heuck C, Wolthers OD, Kollerup G, et al. Adverse effects of inhaled budesonide on growth and collagen turn-over in children with asthma: a double-blind comparison of once-daily versus twice-daily administration. J Pediatr 1998; 133: 608–12
Simons FER. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. Canadian Beclomethasone Dipropionate-Salmeterol Xinafoate Study Group. N Engl J Med 1997; 337: 1659–65
McCowan C, Neville RG, Thomas GE, et al. Effects of asthma and its treatment on growth: four year follow-up of cohort of children from general practices in Tayside, Scotland. BMJ 1998; 316: 668–72
Skoner DP, Szefler SJ, Welch M, et al. Longitudinal growth in infants and young children treated with budesonide inhalation suspension for persistent asthma. J Allergy Clin Immunol 2000; 105: 259–68
Sharek PJ, Bergman DA. The effect of inhaled steroids on linear growth of children with asthma: a meta-analysis. Pediatrics 2000; 106(1): E8
Allen DB, Mullen ML, Mullen B. A meta-analysis of the effect of oral and inhaled corticosteroids on growth. J Allergy Clin Immunol 1994; 93(6): 967–75
American Academy of Allergy, Asthma and Immunology. Pediatric asthma — promoting best practice: guide for managing asthma in children. Milwaukee (WI): The American Academy of Allergy Asthma and Immunology Inc, 1999
Doull IJ, Freezer NJ, Holgate ST. Growth of prepubertal children with mild asthma treated with inhaled beclomethasone dipropionate. Am J Respir Crit Care Med 1995; 151: 1715–9
Verberne AA, Frost C, Roorda RJ, et al. One year treatment with salmeterol compared with beclomethasone in children with asthma. The Dutch Paediatric Asthma Study Group. Am J Respir Crit Care Med 1997; 156: 688–95
Boe J, Bakke P, Rodolen T, et al. High-dose inhaled steroids in asthmatics: moderate efficacy gain and suppression of the hypothalamic-pituitary-adrenal (HPA) axis. Eur Respir J 1994; 7: 2179–84
Ribeiro LB. Budesonide: safety and efficacy aspects of its long-term use in children. Pediatr Allergy Immunol 1993; 4: 73–8
Agertoft L, Pedersen S. Effect of long-term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Respir Med 1994; 88: 373–81
The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000; 343: 1054–63
Norjavara E, Gerhardsson de Vermer M, Lindmark B, et al. Reduced height in Swedish man with asthma at the age of conscription for military service. J Pediatr 2000; 6: 25–9
Doull IJ, Campbell MJ, Holgate ST. Duration of growth suppressive effect of regular inhaled corticosteroids. Arch Dis Child 1998; 78: 172–3
Behrman RE, Kliegman RM, Jenson HB, editors. Nelson textbook of pediatrics. Philadelphia (PA): WB Saunders Company, 2000
Wilson JD, Foster DW, Kronenberg HM, et al., editors. Williams textbook of endocrinology. 9th ed. Philadelphia (PA): Saunders Company, 1998
Kannisto S, Korppi M, Remes K, et al. Adrenal suppression, evaluated by a low dose adrenocorticotropin test, and growth in asthmatic children treated with inhaled steroids. J Clin Endocrinol Metab 2000; 85: 652–7
Hartzband PI, Van Herle AJ, Sorger L, et al. Assessment of hypothalamic pituitary adrenal axis dysfunction comparison of ACTH stimulation, insulin hypoglycemia and metyrapone. J Endocrinol Invest 1988; 11: 769–76
Stewart PM, Corrie J, Seckl JR, et al. A rational approach for assessing the HPA axis. Lancet 1998; I: 1208–10
Bisgaard H, Damkjaer M, Nielsen M, et al. Adrenal function in children with bronchial asthma treated with beclomethasone dipropionate or budesonide. J Allergy Clin Immunol 1988; 81: 1088–95
Broide J, Soferman R, Kivity S, et al. Low-dose adrenocorticotropin test reveals impaired adrenal function in patients taking inhaled corticosteroids. J Clin Endocrinol Metab 1995; 80(4): 1243–6
Barnes PJ, Pedersen S. Efficacy and safety of inhaled corticosteroids in asthma. Am Rev Respir Dis 1993; 148: S1–17
Volovitz B, Amir J, Malik H, et al. Growth and pituitary-adrenal function in children with severe asthma treated with inhaled budesonide. N Engl J Med 1993; 329: 1703–8
Clark DJ, Lipworth BJ, Clark RA. Adrenal suppression with inhaled budesonide and fluticasone propionate given by large volume spacer to asthmatic children. Thorax 1996; 51: 941–3
Goldberg S, Algur N, Levi M, et al. Adrenal suppression among asthmatic children receiving chronic therapy with inhaled corticosteroids with and without spacer device. Ann Allergy Asthma Immunol 1996; 76(3): 234–8
Ninan TK, Reid IW, Carter PE, et al. Effective high doses of inhaled corticosteroids on adrenal function in children with severe persistent asthma. Thorax 1993; 48: 599–602
Brown PH, Blundell G, Greening A, et al. Screening for HPA axis suppression in asthmatics taking high dose inhaled glucocorticosteroids. Respir Med 1991; 85: 511–22
Wolthers OD, Pedersen S. Measures of systemic activity of inhaled corticosteroids in children: a comparison of urine cortisol excretion and knemometry. Respir Med 1995; 89: 347–9
Patel L, Wales JK, Kibirige MS, et al. Symptomatic adrenal insufficiency during inhaled corticosteroid treatment. Arch Dis Child 2001; 85: 330–4
Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000; 343: 332–6
Acknowledgements
We acknowledge Mrs Catriona Cameron for her revision of the English text. The authors have provided no information on sources of funding or on conflicts of interest directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salvatoni, A., Piantanida, E., Nosetti, L. et al. Inhaled Corticosteroids in Childhood Asthma. Pediatr-Drugs 5, 351–361 (2003). https://doi.org/10.2165/00128072-200305060-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00128072-200305060-00001