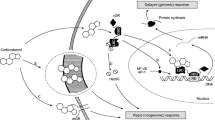
Abstract
International guidelines provide recommendations for a stepwise approach to the management of asthma in children 0–4 years old, 5–11 years old, and adolescents who are treated as adults. Therapy is aimed at two domains of control: current impairment and future risk. The long-term controller medications, inhaled corticosteroids (ICSs), ICSs in combination with long-acting β2 agonists, leukotriene receptor antagonists, and immunomodulators, exhibit different efficacies for these domains. The risk:benefit ratios of the available medications need to be carefully assessed. This review briefly presents the benefits and the potential risks of available asthma medications in children to assist the practitioner in the optimal use of asthma medications. Specifically, the systemic activity of the ICSs and how to minimize their effects on growth and adrenal activity are reviewed as well as other potential adverse effects. Dosing strategies such as intermittent therapy are also assessed.
Similar content being viewed by others
References
Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2014. http://www.ginasthma.org. Accessed Nov 29 2016.
Akinbami LJ, Simon AE, Rossen LM. Changing trends in asthma prevalence among children. Pediatrics. 2016;137(1):e20152354. doi:10.1542/peds.2015-2354.
National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. Bethesda: US Department of Health and Human Services; 2007.
Kaiser SV, Huynh T, Bacharier LB, Rosenthal JL, Bakel LA, Parkin PC, et al. Preventing exacerbations in preschoolers with recurrent wheeze: a meta-analysis. Pediatrics. 2016;137(6):e20154496.
Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–97.
Chauhan BF, Chartrand C, Ni Chroinin M, Milan SJ, Ducharme FM. Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev. 2015;11:CD007949.
Lemanske RF Jr, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, Childhood Asthma Research and Education (CARE) Network of the National Heart, Lung, and Blood Institute, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362:975–85.
Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;5:CD002314.
Knorr B, Franchi LM, Bisgaard H, Vermeulen JH, LeSouef P, Santanello N, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics. 2001;108(3):E48.
van Adelsberg J, Moy J, Wei LX, Tozzi CA, Knorr B, Reiss TF. Safety, tolerability, and exploratory efficacy of montelukast in 6- to 24-month-old patients with asthma. Curr Med Res Opin. 2005;21:971–9.
Brodlie M, Gupta A, Rodriguez-Martinez CE, Castro-Rodriguez JA, Ducharme FM, McKean MC. Leukotriene receptor antagonists as maintenance and intermittent therapy for episodic viral wheeze in children. Cochrane Database Syst Rev. 2015;10:CD008202.
Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–15.
Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136:1476–85.
Kelly HW, Nelson HS. Potential adverse effects of the inhaled corticosteroids. J Allergy Clin Immunol. 2003;112:469–78.
Pedersen S. Clinical safety of inhaled corticosteroids for asthma in children: an update of long-term trials. Drug Saf. 2006;29:599–612.
Fuhlbrigge AL, Kelly HW. Inhaled corticosteroids in children: effects on bone mineral density and growth. Lancet Respir Med. 2014;2(6):487–96.
Pruteanu AI, Chauhan BF, Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: dose-response effects on growth. Cochrane Database Syst Rev. 2014;7:CD009878.
Kapadia CR, Nebesio TD, Myers SE, Willi S, Miller BS, Allen DB, Drugs and Therapeutics Committee of the Pediatric Endocrine Society, et al. Endocrine effects of inhaled corticosteroids in children. JAMA Pediatr. 2016;170(2):163–70.
Raissy HH, Kelly HW, Harkins M, Szefler SJ. Inhaled corticosteroids in lung diseases. Am J Respir Crit Care Med. 2013;187(8):798–803.
Allen DB. Inhaled corticosteroids and growth: still an issue after all these years. J Pediatr. 2015;166(2):463–9.
Kelly HW. Comparison of inhaled corticosteroids: an update. Ann Pharmacother. 2009;43:519–27.
Daley-Yates PT. Inhaled corticosteroids: potency, dose equivalence and therapeutic index. Br J Clin Pharmacol. 2015;80(3):372–80.
Bensch GW, Greos LS, Gawchik S, Kpamegan E, Newman KB. Linear growth and bone maturation are unaffected by 1 year of therapy with inhaled flunisolide hydrofluoroalkane in prepubescent children with mild persistent asthma: a randomized, double-blind, placebo-controlled trial. Ann Allergy Asthma Immunol. 2011;107(4):323–9.
Covar RA, Fuhlbrigge AL, Williams P, Kelly HW, the Childhood Asthma Management Program Research Group. The Childhood Asthma Management Program (CAMP): contributions to the understanding of therapy and the natural history of childhood asthma. Curr Respir Care Rep. 2012;1(4):243–50.
Krishnan JA, Bender BG, Wamboldt FS, Szefler SJ, Adkinson NF Jr, Zeiger RS, Adherence Ancillary Study Group, et al. Adherence to inhaled corticosteroids: an ancillary study of the Childhood Asthma Management Program clinical trial. J Allergy Clin Immunol. 2012;129(1):112–8.
Kelly HW, Sternberg AL, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS, CAMP Research Group, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367(10):904–12.
Bisgaard H, Allen D, Milanowski J, Kalev I, Willits L, Davies P. Twelve-month safety and efficacy of inhaled fluticasone propionate in children aged 1 to 3 years with recurrent wheezing. Pediatrics. 2004;113:e87–94.
Guilbert TW, Mauger DT, Allen DB, Zeiger RS, Lemanske RF Jr, Szefler SJ, Childhood Asthma Research and Education Network of the National Heart, Lung, and Blood Institute, et al. Growth of preschool children at high risk for asthma 2 years after discontinuation of fluticasone. J Allergy Clin Immunol. 2011;128(956–63):e1–7.
Blake K, Mehta R, Spencer T, Kunka RL, Hendeles L. Bioavailability of inhaled fluticasone propionate via chambers/masks in young children. Eur Respir J. 2012;39:97–103.
Elmallah MK, Khan Y, Hochhaus G, Shuster JJ, Hendeles L. Systemic exposure to fluticasone MDI delivered through antistatic chambers. J Allergy Clin Immunol. 2011;128(1113–15):e1–3.
Zeiger RS, Mauger D, Bacharier LB, Guilbert TW, Martinez FD, Lemanske RF Jr, CARE Network of the National Heart, Lung, and Blood Institute, et al. Daily or intermittent budesonide in preschool children with recurrent wheezing. N Engl J Med. 2011;365:1990–2001.
Visser MJ, van der Veer E, Postma DS, Arends LR, de Vries TW, Brand PL, et al. Side-effects of fluticasone in asthmatic children: no effects after dose reduction. Eur Respir J. 2004;24:420–5.
Ducharme FM, Lemire C, Noya FJ, Davis GM, Alos N, Leblond H, et al. Preemptive use of high-dose fluticasone for virus-induced wheezing in young children. N Engl J Med. 2009;360:339–53.
Tse SM, Kelly HW, Litonjua AA, Van Natta ML, Weiss ST, Tantisira KG, Childhood Asthma Management Program Research Group. Corticosteroid use and bone mineral accretion in children with asthma: effect modification by vitamin D. J Allergy Clin Immunol. 2012;130(53–60):e4.
Zöllner EW. Hypothalamic–pituitary–adrenal axis suppression in asthmatic children on inhaled corticosteroids: part 1. Which test should be used? Pediatr Allergy Immunol. 2007;18:401–9.
Zöllner EW, Lombard CJ, Galal U, Hough S, Irusen EM, Weinberg E. Hypothalamic-pituitary-adrenal axis suppression in asthmatic school children. Pediatrics. 2012;130(6):e1512–9.
Zöllner EW, Lombard CJ, Galal U, Hough S, Irusen EM, Weinberg E. Screening for hypothalamic–pituitary–adrenal axis suppression in asthmatic children remains problematic: a cross-sectional study. BMJ Open. 2013;3(8):e002935.
Molimard M, Girodet PO, Pollet C, Fourrier-Reglat A, Davelou A, Haramburu F, et al. Inhaled corticosteroids and adrenal insufficiency: prevalence and clinical presentation. Drug Saf. 2008;31:769–74.
Stockmann C, Fassl B, Gaedigk R, Nkoy F, Uchida DA, Monson S, et al. Fluticasone propionate pharmacogenetics: CYP3A4*22 polymorphism and pediatric asthma control. J Pediatr. 2013;162(6):1222–7.
Cavkaytar O, Vuralli D, Yilmaz EA, Buyuktiryaki B, Soyer O, Sahiner UM, et al. Evidence of hypothalamic–pituitary–adrenal axis suppression during moderate-to-high-dose inhaled corticosteroid use. Eur J Pediatr. 2015;174:1421–31.
Wang JJ, Rochtchina E, Tan AG, Cumming RG, Leeder SR, Mitchell P. Use of inhaled and oral corticosteroids and the long-term risk of cataract. Ophthalmology. 2009;116(4):652–7.
Raissy HH, Sternberg AL, Williams P, Jacobs A, Kelly HW, CAMP Research Group. Risk of cataracts in the Childhood Asthma Management Program Cohort. J Allergy Clin Immunol. 2010;126:389–92.
McMahon AW, Levenson MS, McEvoy BW, Mosholder AD, Murphy D. Age and risks of FDA-approved long-acting β2-adrenergic receptor agonists. Pediatrics. 2011;128(5):e1147–54.
Chowdhury BA, Seymour SM, Levenson MS. Assessing the safety of adding LABAs to inhaled corticosteroids for treating asthma. N Engl J Med. 2011;364:2473–5.
Stempel DA, Szefler SJ, Pedersen S, Zeiger RS, Yeakey AM, Lee LA, VESTRI Investigators, et al. Safety of adding salmeterol to fluticasone propionate in children with asthma. N Engl J Med. 2016;375:840–9.
Stempel DA, Raphiou IH, Kral KM, Yeakey AM, Emmett AH, Prazma CM, AUSTRI Investigators, et al. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med. 2016;374:1822–30.
Peters SP, Bleecker ER, Canonica GW, Park YB, Ramirez R, Hollis S, et al. Serious asthma events with budesonide plus formoterol vs. budesonide alone. N Engl J Med. 2016;375:850–60.
US Food and Drug Administration. Early communication about an ongoing safety review of montelukast (SINGULAIR) http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm095019.htm. Accessed 10 Aug 2009.
US Food and Drug Administration. Update of safety review: follow-up to the March 27, 2008, communication about the ongoing safety review of montelukast (Singulair), January 13, 2009. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm079523.htm. Accessed 10 Aug 2009.
Philip G, Hustad C, Noonan G, Malice M-P, Ezekowitz A, Reiss TF, et al. Reports of suicidality in clinical trials of montelukast. J Allergy Clin Immunol. 2009;124:691–6.
Philip G, Hustad CM, Malice M-P, Noonan G, Ezekowitz A, Reiss TF, et al. Analysis of behavior-related adverse experiences in clinical trials of montelukast. J Allergy Clin Immunol. 2009;124:699–706.
Wallerstedt SM, Brunlöf G, Sundström A, Eriksson AL. Montelukast and psychiatric disorders in children. Pharmacoepidemiol Drug Saf. 2009;18(9):858–64.
Cox L, Lieberman P, Wallace D, Simons FE, Finegold I, Platts-Mills T, et al. American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma & Immunology Omalizumab-Associated Anaphylaxis Joint Task Force follow-up report. J Allergy Clin Immunol. 2011;128(1):210–2.
Zhang L, Axelsson I, Chung M, Lau J. Dose response of inhaled corticosteroids in children with persistent asthma: a systematic review. Pediatrics. 2011;127:129–38.
Fitzpatrick AM, Jackson DJ, Mauger DT, Boehmer SJ, Phipatanakul W, Sheehan WJ, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol. 2016. doi:10.1016/j.jaci.2016.09.028.
Panickar J, Lakhanpaul M, Lambert PC, Kenia P, Stephenson T, Smyth A, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360:329–38.
Beigelman A, Zeiger RS, Kelly HW, Bacharier LB, Childhood Asthma Research and Education Network of National Heart, Lung, and Blood Institute. The challenge of treating preschool wheezing episodes: the need for evidence-based approaches. J Allergy Clin Immunol. 2014;133(4):1016–7.
Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, National Heart, Lung, and Blood Institute’s AsthmaNet, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314(19):2034–44.
Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Pedersen TM, Vinding RK, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4(1):19–26.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with Ethical Standards
No sources of funding were used to assist in the preparation of this manuscript.
Conflict of interest
H. William Kelly discloses the following activities: he was the Principal Investigator for the University of New Mexico site of the CAMP (Childhood Asthma Management Program) study from November 1999 to June 2011; he is currently a member of the independent Joint Oversight Committee for the four FDA-mandated LABA/ICS safety trials by AstraZeneca, GlaxoSmithKline, Merck, and Novartis and the Steering Committee for the Pediatric LABA/ICS study by GlaxoSmithKline. Hengameh Raissy discloses the following activities: she was the principal investigator for the University of New Mexico site for the CAMP Study from July 2011 to June 2012, Childhood Asthma Research Education (CARE) network 3/08-10/10 and AsthmaNet 3/11-present.
Rights and permissions
About this article
Cite this article
Raissy, H.H., Kelly, H.W. Benefits and Risks of Long-Term Asthma Management in Children: Where Are We Heading?. Drug Saf 40, 201–210 (2017). https://doi.org/10.1007/s40264-016-0483-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-016-0483-0