Abstract
Objective: To compare the efficacy, tolerability and local tolerance of diclofenac sodium 0.1% containing hydroxypropylgamma cyclodextrin preserved with benzalkonium chloride 0.005% (Voltaren® Ophtha CD),1 with that of diclofenac sodium 0.1% preserved with thiomersal 0.004% (Voltaren® Ophtha) in the treatment of inflammation after cataract-intraocular lens surgery.
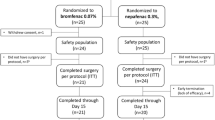
Design and setting: Randomised 2 : 1, double-masked, parallel-group study in six centres in Germany.
Study participants: 299 patients scheduled to undergo phacoemulsification with posterior chamber intraocular lens implantation.
Interventions: Study medications were instilled four times in the 30 minutes before surgery and four times daily from the first postoperative day. Main outcomemeasures: The key efficacy variable was the reduction in anterior chamber flare (photons/millisecond) from day 1 to day 6 to 8. Patients underwent comprehensive ocular examinations, including laser flaremetry (KOWA), preoperatively and postoperatively at days 1, 6 to 8 and 24 to 32.
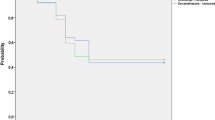
Results: 268 patients (Voltaren® Ophtha CD 177, Voltaren® Ophtha 91) completed the day 6 to 8 visit without any protocol violations. Reduction in the degree of intraocular inflammation with Voltaren® Ophtha CD was equivalent to that achieved with Voltaren® Ophtha at the day 6 to 8 [95% confidence interval (CI) −3.07 to +0.54] and day 24 to 32 (95% CI −1.44 to +1.40) visits. Although there was no significant (p = 0.464) difference between the two study groups in patients’ global assessment of local tolerance at day 24 to 32, ocular discomfort was significantly (p = 0.023) less with Voltaren® Ophtha CD compared with Voltaren® Ophtha.
Conclusions: Voltaren® Ophtha CD was as effective and well tolerated but had less ocular discomfort compared with Voltaren® Ophtha in the treatment of ocular inflammation after phacoemulsification with intraocular lens implantation. This new formulation of diclofenac sodium 0.1% may be used as an alternative to the existing formulations of ophthalmic diclofenac sodium 0.1%.




Similar content being viewed by others
References
Havener WH. Ocular pharmacology. St Louis, CV Mosby, 1994, 408
Polansky JR. Side effects of topical ophthalmic therapy with anti-inflammatory steroids and beta-blockers. Curr Opin Ophthalmol 1992 April; 3 (2): 259–272
Ku EC, Lee W, Kothari HV, Scholer DW. Effect of diclofenac sodium on the arachidonic acid cascade. Am J Med 1986 April; 80 Suppl 4B: 18–23
Roberts CW, Brennan KM. A comparison of topical diclofenac with prednisolone for postcataract inflammation. Arch Ophthalmol 1995 June; 113 (6): 725–727
Stodtmeister R, Marquardt R. A non-steroidal inflammation inhibitor in chronic conjunctivitis. Fortschr Ophthalmol 1986; 83 (2): 199–202
Tauber J, Raizman MB, Ostrov CS, et al. A multi-center comparison of the ocular efficacy and safety of diclofenac 0.1% solution with that of ketorolac 0.5% solution in patients with acute seasonal allergic conjunctivitis. J Ocul Pharmacol Ther 1998 April; 14 (2): 137–145
Szucs PA, Nashed AH, Allegra JR, et al. Safety and efficacy of diclofenac ophthalmic solution in the treatment of corneal abrasions. Ann Emerg Med 2000 Feb; 35 (2): 131–7
Haynes RJ, Walker S, Kirkpatrick JNP. Topical diclofenac relieves pain from corneal rust ring. Eye 1996; 10 (4): 443–6
Sichart U. Voltaren Ophtha sine eye drops in foreign body lesion and keratoconjunctivitis photoelectrica. Spektrum Augenheilkd 1995; 9 (6): 265–9
Sachdev MS, Singh K, Talwar D, et al. Comparative efficacy of diclofenac and flurbiprofen for maintenance of papillary dilatation during cataract surgery. Ophthalmic Surg 1994 Feb; 25 (2): 92–4
Tutton MK, Cherry PMH, Sunder Raj PS, Fsadni MG. Efficacy and safety of topical diclofenac in reducing ocular pain after excimer photorefractive keratectomy. J Cataract Refract Surg 1996 June; 22 (5): 536–541
Epstein RL, Laurence EP. Effect of topical diclofenac solution on discomfort after radial keratotomy. J Cataract Refract Surg 1994 July; 20 (4): 378–380
Morton NS, Benham SW, Lawson RA, et al. Diclofenac vs oxybuprocaine eye drops for analgesia in paediatric strabismus surgery. Paediatr Anaesth 1997; 7 (3): 221–6
Diestelhorst M, Thull D, Krieglstein GK. The effect of argon laser trabeculoplasty on the blood-aqueous barrier and intraocular pressure in human glaucomatous eyes treated with diclofenac 0.1%. Graefes Arch Clin Exp Ophthalmol 1995 Sept; 233 (9): 559–562
Othenin-Girard P, Tritten JJ, Pittet N, et al. Dexamethasone versus diclofenac sodium eye drops to treat inflammation after cataract surgery. J Cataract Refract Surg 1994 Jan; 20 (1): 9–12
Kraff MC, Martin RG, Neumann AC, Weinstein AJ. Efficacy of diclofenac sodium ophthalmic solution versus placebo in reducing inflammation following cataract extraction and posterior chamber lens implantation. J Cataract Refract Surg 1994 March; 20 (2): 138–144
Diestelhorst M, Schmidl B, Konen W, et al. Efficacy and tolerance of diclofenac sodium 0.1%, flurbiprofen 0.03%, and indomethacin 1.0% in controlling postoperative inflammation. J Cataract Refract Surg 1996; 22 Suppl. 1: 788–93
Demco TA, Sutton H, Demco CJ, Raj PS. Topical diclofenac sodium compared with prednisolone acetate after phacoemulsification- lens implant surgery. Eur J Ophthalmol 1997 July–Sept; 7 (3): 236–240
Lesnoni G, Coppe AM, Manni G, et al. Analgesic effect of topical diclofenac versus betamethasone after posterior segment surgery. Retina 1995; 15 (1): 34–6
Loftssona T, Jarvinen T. Cyclodextrins in ophthalmic drug delivery. Adv Drug Deliv Rev 1999 March; 36 (1): 59–79
Suhonen P, Jarvinen T, Lehmussaari K, Reunamaki T, Urtti A. Ocular absorption and irritation of pilocarpine prodrug is modified with buffer, polymer, and cyclodextrin in the eye drop. Pharm Res 1995 April; 12 (4): 529–533
Avci R, Erturk H, Ozcetin H. The effect of diclofenac sodium on postoperative inflammation. Eur J Implant Ref Surg 1993; 5 (1): 68–70
Loftsson T, Fridriksdottir H, Stefansson E, Thorisdottie S, Guomundsson O, Sigthorsson T. Topically effective ocular hypotensive acetazolamide and ethoxyzolamide formulations in rabbits. J Pharm Pharmacol 1994 June; 46 (6): 503–504
Reer O, Bock TK, Mueller BW. In vitro corneal permeability of diclofenac sodium in formulations containing cyclodextrins compared to commercial product voltaren ophtha. J Pharm Sci 1994 Sept; 83 (9): 1345–1349
Zemtsov A, Bolton GG. Thiomerosal-induced bullous contact dermatitis. Contact Dermatitis 1994 Jan; 30 (1): 57
Wilson-Holt N, Dart JKG. Thiomersal keratoconjunctivitis, frequency, clinical spectrumand diagnosis. Eye 1989; 3 (5): 581–7
Tosti A, Tosti G. Thiomerosal: a hidden allergen in ophthalmology. Contact Dermatitis 1988 May; 18 (5): 268–73
Acknowledgements
This study was supported in part by a grant from Novartis Ophthalmics AG, Buelach, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Clinical Grades of Visual Acuity, Ocular Discomfort, Conjunctival Hyperaemia, and Anterior Chamber Flare and Cells
Visual acuity (measured with a pinhole and a Snellen chart at 6 meters under standard lighting conditions)
-
0:
<0.1
-
1:
0.1–0.2
-
2:
0.3–0.4
-
3:
0.5–0.7
-
4:
>0.7
Ocular discomfort (includes superficial pain in particular but also foreign body/gritty sensation, itching, burning and other forms of nonspecific discomfort; grade 4 was to be recorded as an adverse event)
-
0:
absent
-
1:
mild (present but not interfering with daily life)
-
2:
moderate (distressing but not interfering with daily life)
-
3:
severe (very distressing and interfering with daily life but can continue in the study)
-
4:
intolerable (very distressing and interfering with daily life and cannot continue in the study)
Conjunctival hyperaemia
-
0:
absent
-
1:
pale reddish colour of bulbar conjunctiva involving fine conjunctival vessels
-
2:
red colour of bulbar conjunctiva involving fine conunctival and episcleral vessels
-
3:
bright red colour of bulbar conjunctiva involving conjunctival and episcleral vessels
-
4:
bulbar conjunctival injection involving conjunctival and episcleral vessels, and/or definite chemosis of bulbar conjunctiva
All slit-lamp examinations were conducted under standard conditions: dim room illumination, maximal lamp voltage, smallest aperture (0.3mm on Zeiss and 0.2mm in Haag-Streit slit-lamp), illumination angle of 30 to 45°, magnification of 16× and observation for five seconds.
Anterior chamber flare
-
0:
absent
-
1:
trace (barely detectable)
-
2:
mild intensity (iris and lens details clear)
-
3:
moderate intensity (iris and lens details not clear)
-
4:
strong intensity (iris and lens details not visible at all, fibrin in the anterior chamber)
Anterior chamber cells
-
0:
none
-
1:
1 to 5 cells
-
2:
6 to 15 cells
-
3:
16 to 30 cells
-
4:
>30 cells
Rights and permissions
About this article
Cite this article
Mester, U., Lohmann, C., Pleyer, U. et al. A Comparison of Two Different Formulations of Diclofenac Sodium 0.1% in the Treatment of Inflammation following Cataract-Intraocular Lens Surgery. Drugs R&D 3, 143–151 (2002). https://doi.org/10.2165/00126839-200203030-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00126839-200203030-00001