Abstract
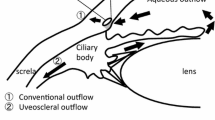
Rho kinase (ROCK1 and ROCK2) is a serine/threonine kinase that serves as an important downstream effector of Rho GTPase, and plays a critical role in regulating the contractile tone of smooth muscle tissues in a calcium-independent manner. Several lines of experimental evidence indicate that modulating ROCK activity within the aqueous humor outflow pathway using selective inhibitors could achieve very significant benefits for the treatment of increased intraocular pressure in patients with glaucoma. The rationale for such an approach stems from experimental data suggesting that both ROCK and Rho GTPase inhibitors can increase aqueous humor drainage through the trabecular meshwork, leading to a decrease in intraocular pressure. In addition to their ocular hypotensive properties, inhibitors of both ROCK and Rho GTPase have been shown to enhance ocular blood flow, retinal ganglion cell survival and axon regeneration. These properties of the ROCK and Rho GTPase inhibitors indicate that targeting the Rho GTPase/ROCK pathway with selective inhibitors represents a novel therapeutic approach aimed at lowering increased intraocular pressure in glaucoma patients.





Similar content being viewed by others
References
Thylefors B, Negrel AD, Pararajasegaram R, et al. Global data on blindness. Bull World Health Organ 1995; 73(1): 115–21
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006; 90(3): 262–7
Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet 2004; 363(9422): 1711–20
Johnson M. What controls aqueous humour outflow resistance? Exp Eye Res 2006; 82(4): 545–57
Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res 2005; 24(5): 612–37
Lutjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res 1999; 18(1): 91–119
Woodward DF, Gil DW. The inflow and outflow of anti-glaucoma drugs. Trends Pharmacol Sci 2004; 25(5): 238–41
Weinreb RN. Uveoscleral outflow: the other outflow pathway. J Glaucoma 2000; 9(5): 343–5
Brubaker RF. Targeting outflow facility in glaucoma management. Surv Ophthalmol 2003; 48Suppl. 1: S17–20
Tan JC, Peters DM, Kaufman PL. Recent developments in understanding the pathophysiology of elevated intraocular pressure. Curr Opin Ophthalmol 2006; 17(2): 168–74
Marquis RE, Whitson JT. Management of glaucoma: focus on pharmacological therapy. Drugs Aging 2005; 22(1): 1–21
Tian B, Geiger B, Epstein DL, et al. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci 2000; 41(3): 619–23
Gong H, Tripathi RC, Tripathi BJ. Morphology of the aqueous outflow pathway. Microsc Res Tech 1996; 33(4): 336–67
Johnstone MA. The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. J Glaucoma 2004; 13(5): 421–38
Francis BA, Alvarado J. The cellular basis of aqueous outflow regulation. Curr Opin Ophthalmol 1997; 8(2): 19–27
Brandt JD, O’Donnell ME. How does the trabecular meshwork regulate outflow? Clues from the vascular endothelium. J Glaucoma 1999; 8(5): 328–39
Ethier CR. The inner wall of Schlemm’s canal. Exp Eye Res 2002; 74(2): 161–72
Wiederholt M, Thieme H, Stumpff F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog Retin Eye Res 2000; 19(3): 271–95
Lutjen-Drecoll E. Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp Eye Res 2005; 81(1): 1–4
Tezel G, Kass MA, Kolker AE, et al. Plasma and aqueous humor endothelin levels in primary open-angle glaucoma. J Glaucoma 1997; 6(2): 83–9
Yorio T, Krishnamoorthy R, Prasanna G. Endothelin: is it a contributor to glaucoma pathophysiology? J Glaucoma 2002; 11(3): 259–70
Tripathi RC, Li J, Chan WF, et al. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res 1994; 59(6): 723–7
Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res 1999; 18(5): 629–67
Epstein DL, Rowlette LL, Roberts BC. Acto-myosin drug effects and aqueous outflow function. Invest Ophthalmol Vis Sci 1999; 40(1): 74–81
Rao PV, Deng PF, Kumar J, et al. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci 2001; 42(5): 1029–37
Yue BY. The extracellular matrix and its modulation in the trabecular meshwork. Surv Ophthalmol 1996; 40(5): 379–90
Johnson DH. Trabecular meshwork and uveoscleral outflow models. J Glaucoma 2005; 14(4): 308–10
deKater AW, Shahsafaei A, Epstein DL. Localization of smooth muscle and nonmuscle actin isoforms in the human aqueous outflow pathway. Invest Ophthalmol Vis Sci 1992; 33(2): 424–9
Rao PV, Deng P, Sasaki Y, et al. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp Eye Res 2005; 80(2): 197–206
Nakamura Y, Hirano S, Suzuki K, et al. Signaling mechanism of TGF-betal-induced collagen contraction mediated by bovine trabecular meshwork cells. Invest Ophthalmol Vis Sci 2002; 43(11): 3465–72
Wiederholt M. Direct involvement of trabecular meshwork in the regulation of aqueous humor outflow. Curr Opin Ophthalmol 1998; 9(2): 46–9
Thieme H, Nuskovski M, Nass JU, et al. Mediation of calcium-independent contraction in trabecular meshwork through protein kinase C and rho-A. Invest Ophthalmol Vis Sci 2000; 41(13): 4240–6
Mettu PS, Deng PF, Misra UK, et al. Role of lysophospholipid growth factors in the modulation of aqueous humor outflow facility. Invest Ophthalmol Vis Sci 2004; 45(7): 2263–71
Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 2003; 83(4): 1325–58
Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem 2001; 276(7): 4527–30
Wettschureck N, Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J Mol Med 2002; 80(10): 629–38
Hartshorne DJ. Myosin phosphatase: subunits and interactions. Acta Physiol Scand 1998; 164(4): 483–93
Pfitzer G. Invited review: regulation of myosin phosphorylation in smooth muscle. J Appl Physiol 2001; 91(1): 497–503
Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci 2001; 22(1): 32–9
Uehata M, Ishizaki T, Satoh H, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997; 389(6654): 990–4
Zhang M, Rao PV. Blebbistatin, a novel inhibitor of myosin II ATPase activity, increases aqueous humor outflow facility in perfused enucleated porcine eyes. Invest Ophthalmol Vis Sci 2005; 46(11): 4130–8
Honjo M, Inatani M, Kido N, et al. A myosin light chain kinase inhibitor, ML-9, lowers the intraocular pressure in rabbit eyes. Exp Eye Res 2002; 75(2): 135–42
Tian B, Kaufman PL, Volberg T, et al. H-7 disrupts the actin cytoskeleton and increases outflow facility. Arch Ophthalmol 1998; 116(5): 633–43
Bill A, Lutjen-Drecoll E, Svedbergh B. Effects of intracameral Na2EDTA and EGTA on aqueous outflow routes in the monkey eye. Invest Ophthalmol Vis Sci 1980; 19(5): 492–504
Ethier CR, Read AT, Chan DW. Effects of latrunculin-B on outflow facility and trabecular meshwork structure in human eyes. Invest Ophthalmol Vis Sci 2006; 47(5): 1991–8
Johnson DH. The effect of cytochalasin D on outflow facility and the trabecular meshwork of the human eye in perfusion organ culture. Invest Ophthalmol Vis Sci 1997; 38(13): 2790–9
Kaufman PL, Erickson KA. Cytochalasin B and D dose-outflow facility response relationships in the cynomolgus monkey. Invest Ophthalmol Vis Sci 1982; 23(5): 646–50
Epstein DL, Freddo TF, Bassett-Chu S, et al. Influence of ethacrynic acid on outflow facility in the monkey and calf eye. Invest Ophthalmol Vis Sci 1987; 28(12): 2067–75
Ethier CR, Coloma FM. Effects of ethacrynic acid on Schlemm’s canal inner wall and outflow facility in human eyes. Invest Ophthalmol Vis Sci 1999; 40(7): 1599–607
Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420(6916): 629–35
Burridge K, Wennerberg K. Rho and Rac take center stage. Cell 2004; 116(2): 167–79
Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans 2005; 33 (Pt 5): 891–5
Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev 1997; 11(18): 2295–322
Cernuda-Morollon E, Ridley AJ. Rho GTPases and leukocyte adhesion receptor expression and function in endothelial cells. Circ Res 2006; 98(6): 757–67
Chitaley K, Weber D, Webb RC. RhoA/Rho-kinase, vascular changes, and hypertension. Curr Hypertens Rep 2001; 3(2): 139–44
Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daaml. Cell 2001; 107(7): 843–54
Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev 2005; 85(4): 1159–204
Sah VP, Seasholtz TM, Sagi SA, et al. The role of Rho in G protein-coupled receptor signal transduction. Annu Rev Pharmacol Toxicol 2000; 40: 459–89
Riobo NA, Manning DR. Receptors coupled to heterotrimeric G proteins of the G12 family. Trends Pharmacol Sci 2005; 26(3): 146–54
Coso OA, Teramoto H, Simonds WF, et al. Signaling from G protein-coupled receptors to c-Jun kinase involves beta gamma subunits of heterotrimeric G proteins acting on a Ras and Rac1-dependent pathway. J Biol Chem 1996; 271(8): 3963–6
Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci 2001; 22(7): 368–76
Dutt P, Nguyen N, Toksoz D. Role of Lbc RhoGEF in Galphal2/13-induced signals to Rho GTPase. Cell Signal 2004; 16(2): 201–9
Tanabe S, Kreutz B, Suzuki N, et al. Regulation of RGS-RhoGEFs by Galphal2 and Galphal3 proteins. Methods Enzymol 2004; 390: 285–94
Cox AD, Der CJ. Protein prenylation: more than just glue? Curr Opin Cell Biol 1992; 4(6): 1008–16
Casey PJ, Seabra MC. Protein prenyltransferases. J Biol Chem 1996; 271(10): 5289–92
Rikitake Y, Liao JK. Rho GTPases, statins, and nitric oxide. Circ Res2005; 97(12): 1232–5
Budzyn K, Marley PD, Sobey CG. Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol Sci 2006; 27(2): 97–104
Fritz G, Kaina B. Rho GTPases: promising cellular targets for novel anticancer drugs. Curr Cancer Drug Targets 2006; 6(1): 1–14
Nakagami H, Jensen KS, Liao JK. A novel pleiotropic effect of statins: prevention of cardiac hypertrophy by cholesterol-independent mechanisms. Ann Med 2003; 35(6): 398–403
Kumar J, Rao PV, Rowlette LL, et al. Rho GTPase-mediated actin cytoskeletal reorganization in outflow pathway cells may modulate outflow function. Invest Ophthalmol Vis Sci 1999; 40: 3534
Liu X, Hu Y, Filla MS, et al. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol Vis 2005; 11: 1112–21
Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 2006; 68: 345–74
Lang P, Gesbert F, Delespine-Carmagnat M, et al. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. Embo J 1996; 15(3): 510–9
Murthy KS, Zhou H, Grider JR, et al. Inhibition of sustained smooth muscle contraction by PKA and PKG preferentially mediated by phosphorylation of RhoA. Am J Physiol Gastrointest Liver Physiol 2003; 284(6): G1006–16
Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2003; 284(6): L972–80
Williams J, Bogwu J, Oyekan A. The role of the RhoA/Rho-kinase signaling pathway in renal vascular reactivity in endothelial nitric oxide synthase null mice. J Hypertens 2006; 24(7): 1429–36
Musicki B, Burnett AL. eNOS function and dysfunction in the penis. Exp Biol Med (Maywood) 2006; 231(2): 154–65
Shiga N, Hirano K, Hirano M, et al. Long-term inhibition of RhoA attenuates vascular contractility by enhancing endothelial NO production in an intact rabbit mesenteric artery. Circ Res 2005; 96(9): 1014–21
Witteck A, Yao Y, Fechir M, et al. Rho protein-mediated changes in the structure of the actin cytoskeleton regulate human inducible NO synthase gene expression. Exp Cell Res 2003; 287(1): 106–15
Ming XF, Viswambharan H, Barandier C, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol 2002; 22(24): 8467–77
Kraynack NC, Corey DA, Elmer HL, et al. Mechanisms of NOS2 regulation by Rho GTPase signaling in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2002; 283(3): L604–11
Bivalacqua TJ, Champion HC, Usta MF, et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A 2004; 101(24): 9121–6
Neufeld AH, Dueker DK, Vegge T, et al. Adenosine 3′,5′-monophosphate increases the outflow of aqueous humor from the rabbit eye. Invest Ophthalmol 1975; 14(1): 40–2
Neufeld AH. Influences of cyclic nucleotides on outflow facility in the vervet monkey. Exp Eye Res 1978; 27(4): 387–97
Kee C, Kaufman PL, Gabelt BT. Effect of 8-Br cGMP on aqueous humor dynamics in monkeys. Invest Ophthalmol Vis Sci 1994; 35(6): 2769–73
Kaufman PL. Adenosine 3′,5′-cyclic-monophosphate and outflow facility in monkey eyes with intact and retrodisplaced ciliary muscle. Exp Eye Res 1987; 44(3): 415–23
Erickson-Lamy KA, Nathanson JA. Epinephrine increases facility of outflow and cyclic AMP content in the human eye in vitro. Invest Ophthalmol Vis Sci 1992; 33(9): 2672–8
Bartels SP, Lee SR, Neufeld AH. Forskolin stimulates cyclic AMP synthesis, lowers intraocular pressure and increases outflow facility in rabbits. Curr Eye Res 1982; 2(10): 673–81
Kotikoski H, Vapaatalo H, Oksala O. Nitric oxide and cyclic GMP enhance aqueous humor outflow facility in rabbits. Curr Eye Res 2003; 26(2): 119–23
Amano M, Chihara K, Kimura K, et al. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 1997; 275(5304): 1308–11
Fujisawa K, Fujita A, Ishizaki T, et al. Identification of the Rho-binding domain of p160ROCK, a Rho-associated coiled-coil containing protein kinase. J Biol Chem 1996; 271(38): 23022–8
Kawano Y, Fukata Y, Oshiro N, et al. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol 1999; 147(5): 1023–38
Nakagawa O, Fujisawa K, Ishizaki T, et al. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett 1996; 392(2): 189–93
Matsui T, Amano M, Yamamoto T, et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. Embo J 1996; 15(9): 2208–16
Nakajima E, Nakajima T, Minagawa Y, et al. Contribution of ROCK in contraction of trabecular meshwork: proposed mechanism for regulating aqueous outflow in monkey and human eyes. J Pharm Sci 2005; 94(4): 701–8
Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J Biol Chem 2001; 276(1): 670–6
Maekawa M, Ishizaki T, Boku S, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 1999; 285(5429): 895–8
Koyama M, Ito M, Feng J, et al. Phosphorylation of CPI-17, an inhibitory phosphoprotein of smooth muscle myosin phosphatase, by Rho-kinase. FEBS Lett 2000; 475(3): 197–200
Li L, Eto M, Lee MR, et al. Possible involvement of the novel CPI-17 protein in protein kinase C signal transduction of rabbit arterial smooth muscle. J Physiol 1998; 508 (Pt 3): 871–81
Kitazawa T, Eto M, Woodsome TP, et al. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol 2003; 546 (Pt 3): 879–89
Woodsome TP, Eto M, Everett A, et al. Expression of CPI-17 and myosin phosphatase correlates with Ca(2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol 2001; 535 (Pt 2): 553–64
Andersson KE. Erectile physiological and pathophysiological pathways involved in erectile dysfunction. J Urol 2003; 170 (2 Pt 2): S6–13; discussion S-4
Chrissobolis S, Sobey CG. Recent evidence for an involvement of rho-kinase in cerebral vascular disease. Stroke 2006; 37(8): 2174–80
Hirooka Y, Shimokawa H. Therapeutic potential of rho-kinase inhibitors in cardiovascular diseases. Am J Cardiovasc Drugs 2005; 5(1): 31–9
Jin L, Burnett AL. RhoA/Rho-kinase in erectile tissue: mechanisms of disease and therapeutic insights. Clin Sci (Lond) 2006; 110(2): 153–65
Moriyama T, Nagatoya K. The Rho-ROCK system as a new therapeutic target for preventing interstitial fibrosis. Drug News Perspect 2004; 17(1): 29–34
Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov 2005; 4(5): 387–98
Mukai Y, Shimokawa H, Matoba T, et al. Involvement of Rho-kinase in hypertensive vascular disease: a novel therapeutic target in hypertension. FASEB J 2001; 15(6): 1062–4
Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol 2006; 290(3): C661–8
Rikitake Y, Liao JK. ROCKs as therapeutic targets in cardiovascular diseases. Expert Rev Cardiovasc Ther 2005; 3(3): 441–51
Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol 2005; 25(9): 1767–75
Wakino S, Kanda T, Hayashi K. Rho/Rho kinase as a potential target for the treatment of renal disease. Drug News Perspect 2005; 18(10): 639–43
Hisaoka T, Yano M, Ohkusa T, et al. Enhancement of Rho/Rho-kinase system in regulation of vascular smooth muscle contraction in tachycardia-induced heart failure. Cardiovasc Res 2001; 49(2): 319–29
Chang S, Hypolite JA, Zderic SA, et al. Increased corpus cavernosum smooth muscle tone associated with partial bladder outlet obstruction is mediated via Rho-kinase. Am J Physiol Regul Integr Comp Physiol 2005; 289(4): R1124–30
Seko T, Ito M, Kureishi Y, et al. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res 2003; 92(4): 411–8
Oi K, Shimokawa H, Hiroki J, et al. Remnant lipoproteins from patients with sudden cardiac death enhance coronary vasospastic activity through upregulation of Rho-kinase. Arterioscler Thromb Vasc Biol 2004; 24(5): 918–22
Jin L, Liu T, Lagoda GA, et al. Elevated RhoA/Rho-kinase activity in the aged rat penis: mechanism for age-associated erectile dysfunction. FASEB J 2006; 20(3): 536–8
Rao PV, Deng P, Maddala R, et al. Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Mol Vis 2005; 11: 288–97
Honjo M, Inatani M, Kido N, et al. Effects of protein kinase inhibitor, HA1077, on intraocular pressure and outflow facility in rabbit eyes. Arch Ophthalmol 2001; 119(8): 1171–8
Aktories K, Mohr C, Koch G. Clostridium botulinum C3 ADP-ribosyltransferase. Curr Top Microbiol Immunol 1992; 175: 115–31
Narumiya S, Morii N. Rho gene products, botulinum C3 exoenzyme and cell adhesion. Cell Signal 1993; 5(1): 9–19
Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 2005; 45: 89–118
Vittitow JL, Garg R, Rowlette LL, et al. Gene transfer of dominant-negative RhoA increases outflow facility in perfused human anterior segment cultures. Mol Vis 2002; 8: 32–44
Song J, Deng PF, Stinnett SS, et al. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Invest Ophthalmol Vis Sci 2005; 46(7): 2424–32
Nagaoka T, Takahashi A, Sato E, et al. Effect of systemic administration of simvastatin on retinal circulation. Arch Ophthalmol 2006; 124(5): 665–70
McGwin G Jr, McNeal S, Owsley C, et al. Statins and other cholesterol-lowering medications and the presence of glaucoma. Arch Ophthalmol 2004; 122(6): 822–6
Honjo M, Tanihara H, Inatani M, et al. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci 2001; 42(1): 137–44
Fukiage C, Mizutani K, Kawamoto Y, et al. Involvement of phosphorylation of myosin phosphatase by ROCK in trabecular meshwork and ciliary muscle contraction. Biochem Biophys Res Commun 2001; 288(2): 296–300
Rosenthal R, Choritz L, Schlott S, et al. Effects of ML-7 and Y-27632 on carbachol- and endothelin-1-induced contraction of bovine trabecular meshwork. Exp Eye Res 2005; 80(6): 837–45
Cellini M, Versura P, Trere D, et al. Effects of endothelin-1 on human trabecular meshwork cell contraction: an in vitro cell culture model. Ophthalmic Res 2005; 37(1): 43–9
Waki M, Yoshida Y, Oka T, et al. Reduction of intraocular pressure by topical administration of an inhibitor of the Rho-associated protein kinase. Curr Eye Res 2001; 22(6): 470–4
Khurana RN, Deng PF, Epstein DL, et al. The role of protein kinase C in modulation of aqueous humor outflow facility. Exp Eye Res 2003; 76(1): 39–47
Koga T, Koga T, Awai M, et al. Rho-associated protein kinase inhibitor, Y-27632, induces alterations in adhesion, contraction and motility in cultured human trabecular meshwork cells. Exp Eye Res 2006; 82(3): 362–70
Tian B, Kaufman PL. Effects of the Rho kinase inhibitor Y-27632 and the phosphatase inhibitor calyculin A on outflow facility in monkeys. Exp Eye Res 2005; 80(2): 215–25
Tamura M, Nakao H, Yoshizaki H, et al. Development of specific Rho-kinase inhibitors and their clinical application. Biochim Biophys Acta 2005; 1754(1–2): 245–52
Sasaki Y, Suzuki M, Hidaka H. The novel and specific Rho-kinase inhibitor (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinoline)sulfonyl]-homopiperazine as a probing molecule for Rho-kinase-involved pathway. Pharmacol Ther 2002; 93(2-3): 225–32
Ishizaki T, Uehata M, Tamechika I, et al. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol 2000; 57(5): 976–83
Delaney Y, Walshe TE, O’Brien C. Vasospasm in glaucoma: clinical and laboratory aspects. Optom Vis Sci 2006; 83(7): 406–14
Grieshaber MC, Flammer J. Blood flow in glaucoma. Curr Opin Ophthalmol 2005; 16(2): 79–83
Stefansson E, Pedersen DB, Jensen PK, et al. Optic nerve oxygenation. Prog Retin Eye Res 2005; 24(3): 307–32
Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol 2003; 48(3): 314–46
Meyer-ter-Vehn T, Sieprath S, Katzenberger B, et al. Contractility as a prerequisite for TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci 2006; 47(11): 4895–904
Mimura F, Yamagishi S, Arimura N, et al. Myelin-associated glycoprotein inhibits microtubule assembly by a Rho-kinase-dependent mechanism. J Biol Chem 2006; 281(23): 15970–9
Alabed YZ, Grados-Munro E, Ferraro GB, et al. Neuronal responses to myelin are mediated by rho kinase. J Neurochem 2006; 96(6): 1616–25
Borisoff JF, Chan CC, Hiebert GW, et al. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci 2003; 22(3): 405–16
Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci 2003; 23(4): 1416–23
Dergham P, Ellezam B, Essagian C, et al. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci 2002; 22(15): 6570–7
Bertrand J, Di Polo A, McKerracher L. Enhanced survival and regeneration of axotomized retinal neurons by repeated delivery of cell-permeable C3-like Rho antagonists. Neurobiol Dis. 2007 Jan; 25(1): 65–72
Bertrand J, Winton MJ, Rodriguez-Hernandez N, et al. Application of Rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats. J Neurosci 2005; 25(5): 1113–21
Kitaoka Y, Kitaoka Y, Kumai T, et al. Involvement of RhoA and possible neuroprotective effect of fasudil, a Rho kinase inhibitor, in NMDA-induced neurotoxicity in the rat retina. Brain Res 2004; 1018(1): 111–8
Gallo G, Letourneau PC. Regulation of growth cone actin filaments by guidance cues. J Neurobiol 2004; 58(1): 92–102
Dickson BJ. Rho GTPases in growth cone guidance. Curr Opin Neurobiol 2001; 11(1): 103–10
Ellis JD, Morris AD, MacEwen CJ. Should diabetic patients be screened for glaucoma? DARTS/MEMO Collaboration. Br J Ophthalmol 1999; 83(3): 369–72
Sommer A. Glaucoma risk factors observed in the Baltimore Eye Survey. Curr Opin Ophthalmol 1996; 7(2): 93–8
Acknowledgments
This review was supported by grants from the NIH/NEI (grants EY013573 and EY12201) to Dr Rao and funding from the Research to Prevent Blindness.
The authors have no conflicts of interest that are directly relevant to the content of this review article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rao, V.P., Epstein, D.L. Rho GTPase/Rho Kinase Inhibition as a Novel Target for the Treatment of Glaucoma. BioDrugs 21, 167–177 (2007). https://doi.org/10.2165/00063030-200721030-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200721030-00004