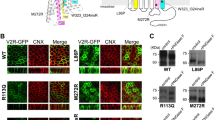
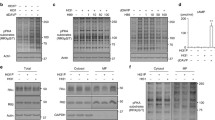
Abstract
The antidiuretic hormone arginine-vasopressin regulates water homeostasis in the human body by binding to its vasopressin type 2 receptor (V2R). Mutations in AVPR2, the gene encoding V2R, lead to the X-linked congenital form of nephrogenic diabetes insipidus (NDI), a disease characterized by the inability to concentrate urine in response to vasopressin; often this involves missense mutations or deletion of one or a few amino acids. In vitro V2R expression studies revealed that the function of most of these receptors is not disturbed, but due to their misfolding, the quality control mechanism of the endoplasmic reticulum (ER) retains these receptors inside the cell, thereby preventing their functioning at the plasma membrane.
This review summarizes our current knowledge on ER retention of V2R mutants, and describes the different approaches that have been undertaken to restore the plasma membrane expression and function of V2R mutants in NDI in vitro and in vivo. The use of cell permeable receptor ligands (called ‘pharmacological chaperones’) appears promising for the treatment of NDI in a subset of patients.




Similar content being viewed by others
References
McKinley MJ, Johnson AK. The physiological regulation of thirst and fluid intake. News Physiol Sci 2004; 19: 1–6
Nonoguchi H, Owada A, Kobayashi N, et al. Immunohistochemical localization of V2 vasopressin receptor along the nephron and functional-role of luminal V2 receptor in terminal inner medullary collecting ducts. J Clin Invest 1995; 96(4): 1768–78
Katsura T, Gustafson CE, Ausiello DA, et al. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol 1997; 41: F816–22
Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 1997; 272: 14800–4
Nielsen S, Digiovanni SR, Christensen EI, et al. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci U S A 1993; 90: 11663–7
Ishibashi K, Sasaki S, Fushimi K, et al. Immunolocalization and effect of dehydration on AQP3, a basolateral water channel of kidney collecting ducts. Am J Physiol 1997; 41: F235–41
Pollak MR. Disturbances of calcium metabolism. In: Brenner BM, editor. The kidney. Philadelphia (PA): W.B. Saunders Company, 2000: 1037–1054
Wingo CS, Weiner ID. Disorders of potassium balance. In: Brenner BM, editor. The kidney. Philapdelphia (PA): W.B. Saunders Company, 2000: 998–1035
Frokiaer J, Marples D, Knepper MA, et al. Bilateral ureteral obstruction downregulates expression of vasopressin-sensitive AQP-2 water channel in rat kidney. Am J Physiol Renal Physiol 1996; 270(4): F657–68
Stone KA. Lithium-induced nephrogenic diabetes insipidus. J Am Board Fam Pract 1999; 12(1): 43–7
Robben JH, Knoers NVAM, Deen PMT. Cell biological aspects of the vasopressin type-2 receptor and aquaporin 2 water channel in nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 2006; 291(2): F257–70
Deen PMT, Verdijk MAJ, Knoers NVAM, et al. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science 1994; 264: 92–5
Kamsteeg EJ, Bichet DG, Konings IBM, et al. Reversed polarized delivery of an aquaporin-2 mutant causes dominant nephrogenic diabetes insipidus. J Cell Biol 2003; 163(5): 1099–109
Mulders SM, Bichet DG, Rijss JPL, et al. An aquaporin-2 water channel mutant which causes autosomal dominant nephrogenic diabetes insipidus is retained in the Golgi complex. J Clin Invest 1998; 102(1): 57–66
Marr N, Bichet DG, Lonergan M, et al. Heteroligomerization of an aquaporin-2 mutant with wild-type aquaporin-2 and their misrouting to late endosomes/lysosomes explains dominant nephrogenic diabetes insipidus. Hum Mol Genet 2002; 11(7): 779–89
Rosenthal W, Seibold A, Antaramian A, et al. Molecular identification of the gene responsible for congenital nephrogenic diabetes insipidus. Nature 1992; 359: 233–5
Knoers N, Monnens LAH. A variant of nephrogenic diabetes-insipidus: V2-receptor abnormality restricted to the kidney. Eur J Pediatr 1991; 150(5): 370–3
Lolait SJ, O’Carroll AM, McBride OW, et al. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature 1992; 357: 336–9
Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 2003; 4(3): 181–91
Schrag JD, Procopio DO, Cygler M, et al. Lectin control of protein folding and sorting in the secretory pathway. Trends Biochem Sci 2003; 28(1): 49–57
Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science 1999; 286(5446): 1882–8
Tatu U, Helenius A. Interactions between newly synthesized glycoproteins, calnexin and a network of resident chaperones in the endoplasmic reticulum. J Cell Biol 1997; 136(3): 555
Kleizen B, Braakman I. Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol 2004; 16(4): 343–9
Ellgaard L, Helenius A. ER quality control: towards an understanding at the molecular level. Curr Opin Cell Biol 2001; 13(4): 431–7
Cabrai CM, Liu Y, Sifers RN. Dissecting glycoprotein quality control in the secretory pathway. Trends Biochem Sci 2001; 26(10): 619–24
Ma Y, Hendershot LM. The unfolding tale of the unfolded protein response. Cell 2001; 107(7): 827–30
Mancias JD, Goldberg J. Exiting the endoplasmic reticulum. Traffic 2005; 6(4): 278–85
Kamhi-Nesher S, Shenkman M, Tolchinsky S, et al. A novel quality control compartment derived from the endoplasmic reticulum. Mol Biol Cell 2001; 12(6): 1711–23
Fullekrug J, Scheiffele P, Simons K. VIP36 localisation to the early secretory pathway. J Cell Sci 1999; 112(17): 2813–21
Hara-Kuge S, Ohkura T, Ideo H, et al. Involvement of VIP36 in intracellular transport and secretion of glycoproteins in polarized madin-darby canine kidney (MDCK) cells. J Biol Chem 2002; 277(18): 16332–9
Czaplewski C, Kazmierkiewicz R, Ciarkowski J. Molecular modeling of the human vasopressin V2 receptor/agonist complex. J Comput Aided Mol Des 1998; 12(3): 275–87
Nephrogenic and neurogenic diabetes insipidus [online]. Available from URL: http://www.medicine.mcgill.ca/nephros [Accessed 2007 Mar 19]
Hermosilla R, Oueslati M, Donalies U, et al. Disease-causing V2 vasopressin receptors are retained in different compartments of the early secretory pathway. Traffic 2004; 5(12): 993–1005
Schulein R, Zuhlke K, Krause G, et al. Functional rescue of the nephrogenic diabetes insipidus-causing vasopressin V2 receptor mutants G185C and R202C by a second site suppressor mutation. J Biol Chem 2001; 276(11): 8384–92
Schulein R, Zuhlke K, Oksche A, et al. The role of conserved extracellular cysteine residues in vasopressin V2 receptor function and properties of two naturally occurring mutant receptors with additional extracellular cysteine residues. FEBS Lett 2000; 466(1): 101–6
Sangkuhl K, Rompier H, Busch W, et al. Nephrogenic diabetes insipidus caused by mutaiton of Tyr205: a key residue of V2 vasopressin receptor function. Hum Mutat 2005; 25(5): 505–13
Oksche A, Dehe M, Schulein R, et al. Folding and cell surface expression of the vasopressin V2 receptor: requirement of the intracellular C-terminus. FEBS Lett 1998; 424(1–2): 57–62
Thielen A, Oueslati M, Hermosilla R, et al. The hydrophobic amino acid residues in the membrane-proximal C tail of the G protein-coupled vasopressin V2 receptor are necessary for transport-competent receptor folding. FEBS Lett 2005; 579(23): 5227–35
Hermosilla R, Schulein R. Sorting functions of the individual cytoplasmic domains of the G protein-coupled vasopressin V(2) receptor in Madin Darby canine kidney epithelial cells. Mol Pharmacol 2001; 60(5): 1031–9
Morello JP, Salahpour A, Petaja-Repo UE, et al. Association of calnexin with wild type and mutant AVPR2 that causes nephrogenic diabetes insipidus. Biochemistry 2001; 40(23): 6766–75
Robben JH, Knoers NVAM, Deen PMT. Characterization of vasopressin V2 receptor mutants in nephrogenic diabetes insipidus in a polarized cell model. Am J Physiol Renal Physiol 2005; 289(2): F265–72
Wuller S, Wiesner B, Loffler A, et al. Pharmacochaperones post-translationally enhance cell surface expression by increasing conformational stability of wild-type and mutant vasopressin V2 receptors. J Biol Chem 2004; 279(45): 47254–63
Bernier V, Morello JP, Zarruk A, et al. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol 2006; 17(1): 232–43
Morello JP, Salahpour A, Laperriere A, et al. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest 2000; 105(7): 887–95
Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones 1996; 1(2): 109–15
Bernier V, Bichet DG, Bouvier M. Pharmacological chaperone action on G-protein-coupled receptors. Curr Opin Pharmacol 2004; 4(5): 528–33
Ulloa-Aguirre A, Janovick JA, Brothers SP, et al. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic 2004; 5(11): 821–37
Robben JH, Sze M, Knoers NVAM, et al. Rescue of vasopressin V2 receptor mutants by chemical chaperones: specificity and mechanism. Mol Biol Cell 2006; 17(1): 379–86
Egan ME, Glockner-Pagel J, Ambrose C, et al. Calcium-pump inhibitors induce functional surface expression of Delta F508-CFTR protein in cystic fibrosis epithelial cells. Nat Med 2002; 8(5): 485–92
Egan ME, Pearson M, Weiner SA, et al. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 2004; 304(5670): 600–2
Delisle BP, Anderson CL, Balijepalli RC, et al. Thapsigargin selectively rescues the trafficking defective LQT2 channels G601S and F805C. J Biol Chem 2003; 278(37): 35749–54
Tan CM, Nickols HH, Limbird LE. Appropriate polarization following pharmacological rescue of V2 vasopressin receptors encoded by X-linked nephrogenic diabetes insipidus alleles involves a conformation of the receptor that also attains mature glycosylation. J Biol Chem 2003; 278(37): 35678–86
Morello JP, Petaja-Repo UE, Bichet DG, et al. Pharmacological chaperones: a new twist on receptor folding. Trends Pharmacol Sci 2000; 21(12): 466–9
Janovick JA, Goulet M, Bush E, et al. Structure-activity relations of successful pharmacologie chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther 2003; 305(2): 608–14
Robben J, Sze M, Knoers N, et al. Functional rescue of vasopressin V2 receptor mutants in MDCK cells by pharmacochaperones: relevance to therapy of nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 2007 Jan; 292(1): F253–F260. Epub 2006 Aug 22
Petaja-Repo UE, Hogue M, Bhalla S, et al. Ligands act as pharmacological chaperones and increase the efficiency of delta opioid receptor maturation. EMBO J 2002; 21(7): 1628–37
Hawtin SR. Pharmacological chaperone activity of SR49059 to functionally recover mis-folded mutations of the vasopressin V1a receptor. J Biol Chem 2006 May 26; 281(21): 14604–14614. Epub 2006 Mar 24
Noorwez SM, Malhotra R, McDowell JH, et al. Retinoids assist the cellular folding of the autosomal dominant retinitis pigmentosa opsin mutant P23H. J Biol Chem 2004; 279(16): 16278–84
Serradeil-Le Gal C, Wagnon J, Valette G, et al. Nonpeptide vasopressin receptor antagonists: development of selective and orally active V1a, V2 and V1b receptor ligands. Prog Brain Res 2002; 139: 197–210
Bernier V, Lagace M, Lonergan M, et al. Functional rescue of the constitutively internalized V2 vasopressin receptor mutant R137H by the pharmacological chaperone action of SR49059. Mol Endocrinol 2004; 18(8): 2074–84
Castro-Fernandez C, Maya-Nunez G, Conn PM. Beyond the signal sequence: protein routing in health and disease. Endocr Rev 2005; 26(4): 479–503
Conn PM, Leanos-Miranda A, Janovick JA. Protein origami: therapeutic rescue of misfolded gene products. Mol Interv 2002; 2(5): 308–16
Schoneberg T, Schulz A, Biebermann H, et al. Mutant G-protein-coupled receptors as a cause of human diseases. Pharmacol Ther 2004; 104(3): 173–206
Bernier V, Lagace M, Bichet DG, et al. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab 2004; 15(5): 222–8
Ficker E, Obejero-Paz CA, Zhao S, et al. The binding site for channel blockers that rescue misprocessed human long QT syndrome type 2 ether-a-gogo-related gene (HERG) mutations. J Biol Chem 2002; 277(7): 4989–98
Gong Q, Jones MA, Zhou Z. Mechanisms of pharmacological rescue of trafficking-defective hERG mutant channels in human long QT syndrome. J Biol Chem 2006; 281(7): 4069–74
Wang Y, Bartlett MC, Loo TW, et al. Specific rescue of CFTR processing mutants using pharmacological chaperones. Mol Pharmacol 2006 Jul; 70(1): 297–302. Epub 2006 Apr 19
Yun J, Schoneberg T, Liu J, et al. Generation and phenotype of mice harboring a nonsense mutation in the V2 vasopressin receptor gene. J Clin Invest 2000; 106(11): 1361–71
Knoers NV, Monnens LL. Nephrogenic diabetes insipidus. Semin Nephrol 1999; 19(4): 344–52
Fujiwara TM, Bichet DG. Molecular biology of hereditary diabetes insipidus. J Am Soc Nephrol 2005; 16(10): 2836–46
Barak LS, Oakley RH, Laporte SA, et al. Constitutive arrestin-mediated desensitization of a human vasopressin receptor mutant associated with nephrogenic diabetes insipidus. Proc Natl Acad Sci U S A 2001; 98(1): 93–8
Ohnishi A, Orita Y, Takagi N, et al. Aquaretic effect of a potent, orally active, nonpeptide V2 antagonist in men. J Pharmacol Exp Ther 1995; 272(2): 546–51
Bennett WM. V2 receptor antagonists in cystic kidney diseases: an exciting step towards a practical treatment. J Am Soc Nephrol 2005; 16(4): 838–9
Bichet DG, Razi M, Lonergan M, et al. Hemodynamic and coagulation responses to 1-desamino[8-D-arginine] vasopressin in patients with congenital nephrogenic diabetes insipidus. N Engl J Med 1988; 318(14): 881–7
Nakamura S, Yamamura Y, Itoh S, et al. Characterization of a novel nonpeptide vasopressin V2-agonist, OPC-51803, in cells transfected human vasopressin receptor subtypes. Br J Pharmacol 2000; 129(8): 1700–6
Petaja-Repo UE, Hogue M, Laperriere A, et al. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem 2001; 276(6): 4416–23
Blankley CJ, Hodges CJ, Klutchko SR, et al. Synthesis and structure-activity relationships of a novel series of non-peptide angiotensin II receptor binding inhibitors specific for the AT2 subtype. J Med Chem 1991; 34(11): 3248–60
Breu V, Clozel M, Burri K, et al. In vitro characterisation of Ro 46-8443, the first non-peptide antagonist selective for the endothelin ETB receptor. FEBS Lett 1996; 383(1–2): 37–41
Buchan KW, Alldus C, Chrisodoulou C, et al. Characterization of three nonpeptide endothelin receptor ligands using human cloned ETA and ETB receptors. Br J Pharmacol 1994; 112(4): 1251–7
vanStraten NCR, vanBerkel THJ, Demont DR, et al. Identification of substituted 6-amino-4-phenyltetrahydroquinoline derivatives: potent antagonists for the follicle-stimulating hormone receptor. J Med Chem 2005; 48(6): 1697–700
Fleck BA, Chen C, Yang W, et al. Molecular interactions of nonpeptide agonists and antagonists with the melanocortin-4 receptor. Biochemistry 2005; 44(44): 14494–508
Grimberg H, Zaltsman I, Lupu-Meiri M, et al. Inverse agonist abolishes desensitization of a constitutively active mutant of thyrotropin-releasing hormone receptor: role of cellular calcium and protein kinase C. Br J Pharmacol 1999; 126(5): 1097–106
Acknowledgments
This project is supported by a grant from the Dutch Kidney Foundation (PC 104) to Dr Deen and the Netherlands Organisation for Scientific Research (NWO; 825.06.010) to Dr Robben.
The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robben, J.H., Deen, P.M. Pharmacological Chaperones in Nephrogenic Diabetes Insipidus. BioDrugs 21, 157–166 (2007). https://doi.org/10.2165/00063030-200721030-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200721030-00003