Abstract
Interferon-β-1b (Betaseron®, Betaferon®) is a non-glycosylated recombinant human interferon-β approved for high-frequency, subcutaneous (SC) administration in the treatment of multiple sclerosis (MS). Its mechanism of action is unknown, but may involve modulation of the autoimmune pathogenic processes of MS.
In a randomized, double-blind trial in patients with relapsing-remitting MS (RRMS), SC interferon-β-1b 250μg (8 million International Units [MIU]) every other day reduced the annual relapse rate and increased the proportion of relapse-free patients compared with placebo. It also reduced relapse severity, hospitalizations, and disease activity assessed by magnetic resonance imaging (MRI), and increased the time to first relapse. Progression of disability showed a trend towards reduction relative to placebo and baseline, but did not reach statistical significance.
SC interferon-β-1b 250μg every other day was shown in a randomized trial to be superior to intramuscular (IM) interferon-β-1a 30μg (6 MIU) once weekly with respect to reductions in relapse-related parameters, disability progression and MRI-assessed disease activity.
In patients with secondary progressive MS (SPMS), SC interferon-β-1b 250μg every other day slowed progression of the disease relative to placebo in one randomized, double-blind trial, but not in another. In both studies, interferon-β-1b 250μg recipients had fewer relapses and less MRI-assessed disease activity than placebo recipients. The difference in primary outcome may reflect differences in patient entry criteria.
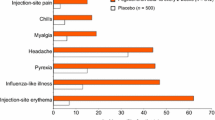
Interferon-β-1b is generally well tolerated and the common adverse events (e.g. injection site reactions, asthenia and an influenza-like symptom complex) are clinically manageable. In a randomized trial, the tolerability of SC interferon-β-1b 250μg every other day was generally similar to that of IM interferon-β-1a 30μg once weekly, except for higher incidences of injection site reactions and neutralizing anti-interferon-β antibodies with SC interferon-β-1b.
Similar content being viewed by others
References
Dhib-Jalbut S. Mechanisms of action of interferons and glatiramer acetate in multiple sclerosis. Neurology 2002 Apr 23; 58(8 Suppl. 4): S3–9
Horowski R. Multiple sclerosis and interferon beta-1b, past, present and future. Clin Neurol Neurosurg 2002 Jul; 104(3): 259–64
Rolak LA. Multiple sclerosis treatment 2001. Neurol Clin 2001 Feb; 19(1): 107–18
Huynh HK, Oger J, Dorovini-Zis K. Interferon-β downregulates interferon-γ- induced class II MHC molecule expression and morphological changes in primary cultures of human brain microvessel endothelial cells. J Neuroimmunol 1995; 60(1-2): 63–73
Genc K, Dona DL, Reder AT. Increased CD80+ B cells in active multiple sclerosis and reversal by interferon β-1b therapy. J Clin Invest 1997 Jun 1; 99(11): 2664–71
Ozenci V, Kouwenhoven M, Huang YM, et al. Multiple sclerosis: levels of interleukin-10-secreting blood mononuclear cells are low in untreated patients but augmented during interferon-β-1b treatment. Scand J Immunol 1999 May; 49(5): 554–61
Nicoletti F, Di Marco R, Patti F, et al. Blood levels of transforming growth factor-beta 1 (TGF-β1) are elevated in both relapsing remitting and chronic progressive multiple sclerosis (MS) patients and are further augmented by treatment with interferon-beta 1b (IFN-β1b). Clin Exp Immunol 1998 Jul; 113(1): 96–9
Brod SA, Marshall GD Jr, Henninger EM, et al. Interferon-β1b treatment decreases tumor necrosis factor-α and increases interleukin-6 production in multiple sclerosis. Neurology 1996 Jun; 46(6): 1633–8
Calabresi PA, Tranquill LR, Dambrosia JM, et al. Increases in soluble VCAM-1 correlate with a decrease in MRI lesions in multiple sclerosis treated with interferon β-1b. Ann Neurol 1997 May; 41(5): 669–74
Prat A, Al-Asmi A, Duquette P, et al. Lymphocyte migration and multiple sclerosis: Relation with disease course and therapy. Ann Neurol 1999; 46(2): 253–6
Uhm JH, Dooley NP, Stuve O, et al. Migratory behavior of lymphocytes isolated from multiple sclerosis patients: effects of interferon β-1b therapy. Ann Neurol 1999 Sep; 46(3): 319–24
Trojano M, Avolio C, Liuzzi GM, et al. Changes of serum sICAM-1 and MMP-9 induced by rIFNβ-1b treatment in relapsing-remitting MS. Neurology 1999 Oct 22; 53(7): 1402–8
Pachner AR. Measurement of antibodies to interferon beta in patients with multiple sclerosis. Arch Neurol 2001 Aug; 58(8): 1299–300
Rice G. The significance of neutralizing antibodies in patients with multiple sclerosis treated with interferon beta. Arch Neurol 2001 Aug; 58(8): 1297–8
The IFNB Multiple Sclerosis Study Group, University of British Columbia MS/ MRI Analysis Group. Neutralizing antibodies during treatment of multiple sclerosis with interferon beta-1b: experience during the first three years. Neurology 1996 Oct; 47(4): 889–94
Polman C, Kappos L, White R, et al. Neutralizing antibodies during treatment of secondary progressive MS with interferon β-1b. Neurology 2003 Jan 14; 60(1): 37–43
USFDA. Medical officer clinical review of the North American study of Betaseron® in the treatment of secondary progressive multiple sclerosis [online]. Available from URL: http://www.fda.gov/cder/biologics/review/ifnbchi031403r2.pdf [Accessed 2003 Oct 15]
Hurwitz B, Polman C. Neutralizing antibodies to interferon beta-1b (Betaferon®/ Betaseron®): real-world data [poster no. P181]. 19th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); 2003 Sep 17–20; Milan
Knobler RL, Greenstein JI, Johnson KP, et al. Systemic recombinant human interferon-β treatment of relapsing-remitting multiple sclerosis: pilot study analysis and six-year follow-up. J Interferon Res 1993 Oct; 13(5): 333–40
Kremenchutzky M. Long-term evolution of anti-IFNb antibodies in IFNb-treated MS patients: the London, Canada, MS Clinic experience. Neurology 2003 Nov; 61Suppl. 5: S29–30
Pachner AR. Anti-IFNβ antibodies in IFNβ-treated MS patients: summary. Neurology 2003 Nov; 61Suppl. 1: S1–5
Khan OA, Dhib-Jalbut SS. Neutralizing antibodies to interferon β-1a and interferon β-1b in MS patients are cross-reactive. Neurology 1998 Dec; 51(6): 1698–702
Bertolotto A, Malucchi S, Milano E, et al. Interferon β neutralizing antibodies in multiple sclerosis: neutralizing activity and cross-reactivity with three different preparations. Immunopharmacology 2000 Jul 20; 48(2): 95–100
Ross C, Clemmesen KM, Svenson M, et al. Immunogenicity of interferon-β in multiple sclerosis patients: influence of preparation, dosage, dose frequency, and route of administration. Ann Neurol 2000; 48(5): 706–12
Chiang J, Gloff CA, Yoshizawa CN, et al. Pharmacokinetics of recombinant human interferon-β ser in healthy volunteers and its effect on serum neopterin. Pharm Res 1993 Apr; 10(4): 567–72
Khan OA, Xia Q, Bever CT, et al. Interferon beta-1b serum levels in multiple sclerosis patients following subcutaneous administration. Neurology 1996 Jun; 46(6): 1639–43
Sturzebecher S, Maibauer R, Heuner A, et al. Pharmacodynamic comparison of single doses of IFN-β1a and IFN-β1b in healthy volunteers. J Interferon Cytokine Res 1999; 19(11): 1257–64
Williams GJ, Witt PL. Comparative study of the pharmacodynamic and pharmacologic effects of Betaseron and AVONEX. J Interferon Cytokine Res 1998 Nov; 18(11): 967–75
The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I: clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993 Apr; 43(4): 655–61
Paty DW, Li DK. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II: MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology 1993 Apr; 43(4): 662–7
The IFNB Multiple Sclerosis Study Group, University of British Columbia MS/ MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology 1995 Jul; 45: 1277–85
Durelli L, Verdun E, Barbero P, et al. Every-other-day Interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet 2002 Apr 27; 359(9316): 1453–60
European Study Group on Interferon β-1b in Secondary Progressive MS. Placebo-controlled multicentre randomised trial of interferon β-1b in treatment of secondary progressive multiple sclerosis. Lancet 1998 Nov 7; 352: 1491–7
Kuhle J, Hardmeier M, D’Hooghe M. Long-term follow-up of the European Study of Interferon beta-1b (EUSPMS) in secondary progressive MS [abstract no. P329, plus poster]. 19th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); 2003 Sep 17–20; Milan, Italy
Goodkin DE, and the North American Study Group on Interferon β-1b in Secondary Progressive MS. Interferon beta-1b in secondary progressive MS: clinical and MRI results of a 3-year randomized controlled trial [abstract no. LBN.004]. 52nd Annual Meeting of the American Academy of Neurology Late Breakers; 2000 Apr 29–May 6; San Diego
Freeman JA, Thompson AJ, Fitzpatrick R, et al. Interferon-β1b in the treatment of secondary progressive MS: impact on quality of life. Neurology 2001 Nov 27; 57(10): 1870–5
Rice GP, Oger J, Duquette P, et al. Treatment with interferon beta-1b improves quality of life in multiple sclerosis. Can J Neurol Sci 1999 Nov; 26(4): 276–82
Chilcott J, McCabe C, Tappenden P, et al. Modelling the cost effectiveness of interferon beta and glatiramer acetate in the management of multiple sclerosis. BMJ 2003; 326(7388): 522–5
Tappenden P, Chilcott J, O’Hagan T, et al. Cost effectiveness of beta interferons and glatiramer acetate in the management of multiple sclerosis: final report to the National Institute for Clinical Excellence [online]. Available from URL: www.nice.org.uk/pdf/msscharrreport.pdf [Accessed 2004 Mar 30]
Kobelt G, Jönsson L, Fredrikson S. Cost-utility of interferon β1b in the treatment of patients with active relapsing-remitting or secondary progressive multiple sclerosis. Eur J Health Econom 2003; 4(1): 50–9
Chiron Corporation, Berlex Laboratories. Betaseron®: interferon beta-1b, US prescribing information [online]. Available from URL: http://www.betaseron.com/preinfo [Accessed 2003 Nov 24]
Durelli L, Ferrero B, Oggero A, et al. Autoimmune events during interferon beta-1b treatment for multiple sclerosis. J Neurol Sci 1999 Jan 1; 162(1): 74–83
Ferrero B, Durelli L, Ecari U, et al. The Betaferon Safety Trial: prospective multicentric monitoring of autoimmunity during interferon-beta-1b (IFNB) therapy for multiple sclerosis (MS) [abstract no. P01.071]. Neurology 1998 Apr; 50(4 Suppl.): 33
Kreisler A, de Seze J, Stojkovic T, et al. Multiple sclerosis, interferon beta and clinical thyroid dysfunction. Acta Neurol Scand 2003 Feb; 107(2): 154–7
Rotondi M, Oliviero A, Profice P, et al. Occurrence of thyroid autoimmunity and dysfunction throughout a nine-month follow-up in patients undergoing interferon-β therapy for multiple sclerosis. J Endocrinol Invest 1998; 21(11): 748–52
Schering AG. Betaferon®: summary of product characteristics, European prescribing information [online]. Available from URL: http://www.emea.eu.int/humandocs/humans/EPAR/Betaferon/Betaferon.htm [Accessed 2003 Oct 15]
Goodin DS. A comparison of the biological response and tolerability between the two high-dose, high-frequency beta interferons in healthy volunteers [poster]. Presented at the 13th European Neurological Society Annual Meeting; 2003 Jun 14–18; Istanbul
Freedman MS, Blumhardt LD, Brochet B, et al. International consensus statement on the use of disease-modifying agents in multiple sclerosis. Mult Scler 2002 Feb; 8(1): 19–23
Lublin FD, Whitaker JN, Eidelman BH, et al. Management of patients receiving interferon beta-1b for multiple sclerosis: report of a consensus conference. Neurology 1996 Jan; 46(1): 12–8
Acknowledgments
The full text article in CNS Drugs 2004; 18 (8): 521–546 was reviewed by: G. Comi, Department of Neurology, Ospedale San Raffaele, Milan, Italy; F. Deisenhammer, Department of Neurology, University of Innsbruck, Innsbruck, Austria; O. Fernández, Institute of Neurosciences, Hospital Regional Universitario Carlos Haya, Malaga, Spain; M. Filippi, Scientific Institute and University San Raffaele, Milan, Italy; R.B. Forbes, Department of Neurology, Royal Victoria Hospital, Belfast, Northern Ireland; M.J. Nuijten, MEDTAP International, Amsterdam, The Netherlands; T. Traboulsee, University of British Columbia, Vancouver, British Columbia, Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
This Spotlight is derived from abstract and summary text of an Adis Drug Evaluation originally published in full in CNS Drugs 2004; 18 (8): 521–546. Reviewers of the original full text article are listed in the Acknowledgments section
Rights and permissions
About this article
Cite this article
McCormack, P.L., Scott, L.J. Spotlight on Interferon-β-1b in Relapsing-Remitting and Secondary Progressive Multiple Sclerosis. BioDrugs 18, 343–347 (2004). https://doi.org/10.2165/00063030-200418050-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200418050-00006