Abstract
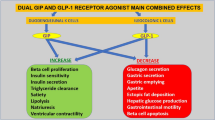
Gastric inhibitory polypeptide (GIP, also called glucose-dependent insulinotropic polypeptide) and glucagon-like peptide-1 (GLP-1) are peptide hormones from the gut that enhance nutrient-stimulated insulin secretion (the ‘incretin’ effect). Judging from experiments in mice with targeted deletions of GIP and GLP-1 receptors, the incretin effect is essential for normal glucose tolerance. In patients with type 2 diabetes mellitus it turns out that the incretin effect is severely impaired or abolished. The explanation seems to be that both the secretion of GLP-1 and the effect of GIP are impaired (whereas both the secretion of GIP and the effect of GLP-1 are near normal). The impaired GLP-1 secretion is probably a consequence of diabetic metabolic disturbances. The known genetic variations in the GIP receptor sequence are not associated with type 2 diabetes mellitus, but a defective insulinotropic effect of GIP may be found in first degree relatives of the patients, suggesting a genetic background for the defect. The molecular nature of the defect is not known and given the close similarity of the two receptors and their signalling, the dissociation of their effects is remarkable. Whereas GLP-1 and its analogues are attractive as therapeutic agents for type 2 diabetes mellitus, analogues of GIP are unlikely to be effective. On the other hand, GIP seems to play an important role in lipid metabolism, promoting the disposal of ingested lipids, and mice with a targeted deletion of the GIP receptor do not become obese when exposed to a high-fat diet. Therefore, antagonistic analogues of GIP may be speculated to have a role in the pharmaceutical management of obesity.
Similar content being viewed by others
References
Brown JC. Gastric inhibitory polypeptide. Monogr Endocrinol 1982; 24: 1–88
McIntyre N, Holdsworth CD, Turner DS. New interpretation of oral glucose tolerance. Lancet 1964; II: 20–1
Zunz E, LaBarre J. Contributions a l’etude des variation physiologiques de la secretion interne de pancreas: relations entre les secretions externe et interne du pancreas. Arch Int Physiol Biochim 1929; 31: 20–44
Ebert R. Gut signals for islet hormone release. Eur J Clin Invest 1990; 20 Suppl. 1: S20–6
Fieseler P, Bridenbaugh S, Nustede R, et al. Physiological augmentation of amino acid-induced insulin secretion by GIP and GLP-I but not by CCK-8. Am J Physiol 1995; 268 (5 Pt 1): E949–55
Baum F, Nauck MA, Ebert R, et al. Role of endogenously released cholecystokinin in determining postprandial insulin levels in man: effects of loxiglumide, a specific cholecystokinin receptor antagonist. Digestion 2001; 53: 189–99
Ahren B, Holst JJ, Efendic S. Antidiabetogenic action of cholecystokinin-8 in type 2 diabetes. J Clin Endocrinol Metab 2000; 85(3): 1043–8
Fehmann HC, Goke R, Goke B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr Rev 1995; 16(3): 390–410
Holst JJ. Glucagon-like peptide 1(GLP-1): an intestinal hormone signalling nutritional abundance, with an unusual therapeutic potential. Trends Endocrinol Metab 1999; 10(6): 229–34
Bell GI, Sanchez-Pescador R, Laybourn PJ, et al. Exon duplication and divergence in the human preproglucagon gene. Nature 1983; 304(5924): 368–71
Mojsov S, Heinrich G, Wilson IB, et al. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem 1986; 261(25): 11880–9
Orskov C, Holst JJ, Knuhtsen S, et al. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology 1986; 119(4): 1467–75
Orskov C, Wettergren A, Holst JJ. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol 1996; 31(7): 665–70
Kreymann B, Williams G, Ghatei MA, et al. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 1987; II(8571): 1300–4
Kolligs F, Fehmann HC, Goke R, et al. Reduction of the incretin effect in rats by the glucagon-like peptide 1 receptor antagonist exendin (9-39) amide. Diabetes 1995; 44(1): 16–9
Wang Z, Wang RM, Owji AA, et al. Glucagon-like peptide-1 is a physiological incretin in rat. J Clin Invest 1995; 95(1): 417–21
Scrocchi LA, Brown TJ, MaClusky N, et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 1996; 2(11): 1254–8
Takeda J, Seino Y, Tanaka K, et al. Sequence of an intestinal cDNA encoding human gastric inhibitory polypeptide precursor. Proc Natl Acad Sci U S A 1987; 84(20): 7005–8
Krarup T. Immunoreactive gastric inhibitory polypeptide. Endocr Rev 1988; 9(1): 122–34
Krarup T, Holst JJ, Larsen KL. Responses and molecular heterogeneity of IR-GIP after intraduodenal glucose and fat. Am J Physiol 1985; 249 (2 Pt 1): E195–200
Usdin TB, Mezey E, Button DC, et al. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 1993; 133(6): 2861–70
Deacon CF, Nauck MA, Meier J, et al. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab 2000; 85(10): 3575–81
Deacon CF, Danielsen P, Klarskov L, et al. Dipeptidyl peptidase IV inhibition reduces the degradation and clearance of GIP and potentiates its insulinotropic and antihyperglycemic effects in anesthetized pigs. Diabetes 2001; 50(7): 1588–97
O’Harte FP, Mooney MH, Flatt PR. NH2-terminally modified gastric inhibitory polypeptide exhibits amino-peptidase resistance and enhanced antihyperglycemic activity. Diabetes 1999; 48(4): 758–65
Kuhn-Wache K, Manhart S, Hoffmann T, et al. Analogs of glucose-dependent insulinotropic polypeptide with increased dipeptidyl peptidase IV resistance. Adv Exp Med Biol 2000; 477: 187–95
Mortensen K, Petersen LL, Orskov C. Colocalization of GLP-1 and GIP in human and porcine intestine. Ann N Y Acad Sci 2000; 921: 469–72
Pederson RA. Gastric inhibitory polypeptide. In: Walsh JH, Dockray GJ, editors. Gut peptides: biochemistry and physiology. New York: Raven Press, 1994: 217–60
Ding WG, Renstrom E, Rorsman P, et al. Glucagon-like peptide I and glucose-dependent insulinotropic polypeptide stimulate Ca2+-induced secretion in rat alpha-cells by a protein kinase A-mediated mechanism. Diabetes 1997; 46(5): 792–800
Dupre J, Ross SA, Watson D, et al. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab 1973; 37: 826–8
Andersen DK, Elahi D, Brown JC, et al. Oral glucose augmentation of insulin secretion: interactions of gastric inhibitory polypeptide with ambient glucose and insulin levels. J Clin Invest 1978; 49: 152–61
Lauritsen KB, Holst JJ, Moody AJ. Depression of insulin release by anti-GIP serum after oral glucose in rats. Scand J Gastroenterol 1981; 16: 417–20
Ebert R, Creutzfeldt W. Influence of gastric inhibitory polypeptide antiserum on glucose-induced insulin secretion in rats. Endocrinology 1982; 111: 1601–6
Tseng CC, Kieffer TJ, Jarboe LA, et al. Postprandial stimulation of insulin release by glucose-dependent insulinotropic polypeptide (GIP): effect of a specific glucose-dependent insulinotropic polypeptide receptor antagonist in the rat. J Clin Invest 1996; 98(11): 2440–5
Miyawaki K, Yamada Y, Yano H, et al. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci U S A 1999; 96(26): 14843–7
Lewis JT, Dayanandan B, Habener JF, et al. Glucose-dependent insulinotropic polypeptide confers early phase insulin release to oral glucose in rats: demonstration by a receptor antagonist. Endocrinology 2000; 141(10): 3710–6
Nauck M, Schmidt WE, Ebert R, et al. Insulinotropic properties of synthetic human gastric inhibitory polypeptide in man: interactions with glucose, phenylalanine, and cholecystokinin-8. J Clin Endocrinol Metab 1989; 69(3): 654–62
Qualmann C, Nauck MA, Holst JJ, et al. Glucagon-like peptide 1 (7-36 amide) secretion in response to luminal sucrose from the upper and lower gut: a study using alpha-glucosidase inhibition (acarbose). Scand J Gastroenterol 1995; 30(9): 892–6
Nauck MA, Bartels E, Orskov C, et al. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab 1993; 76(4): 912–7
Baggio L, Kieffer TJ, Drucker DJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, regulates fasting glycemia and nonenteral glucose clearance in mice. Endocrinology 2000; 141(10): 3703–9
Nauck M, Stockmann F, Ebert R, et al. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29(1): 46–52
Tronier B, Dejgaard A, Andersen T, et al. Absence of incretin effect in obese type 2 and diminished effect in lean type 2 and obese subjects [abstract]. Diabetes Res Clin Pract 1985; Suppl. 1: S568
Orskov C, Vilsboll T, Krarup T, et al. Lack of germ-line mutations in the GIP-coding region of the pro-GIP in type II diabetic patients [abstract]. Diabetes 1999; 48 Suppl. 1: A427
Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 2001; 86(8): 3717–23
Vaag AA, Holst JJ, Volund A, et al. Gut incretin hormones in identical twins discordant for non-insulin-dependent diabetes mellitus (NIDDM)-evidence for decreased glucagon-like peptide 1 secretion during oral glucose ingestion in NIDDM twins. Eur J Endocrinol 1996; 135(4): 425–32
Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993; 91(1): 301–7
Elahi D, McAloon Dyke M, Fukagawa NK, et al. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Regul Pept 1994; 51(1): 63–74
Nauck MA, Kleine N, Orskov C, et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993; 36(8): 741–4
Nauck MA, Holst JJ, Willms B. Glucagon-like peptide 1 and its potential in the treatment of non-insulin-dependent diabetes mellitus. Horm Metab Res 1997; 29(9): 411–6
Fehmann HC, Habener JF. Insulinotropic hormone glucagon-like peptide-I(7-37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology 1992; 130(1): 159–66
Buteau J, Roduit R, Susini S, et al. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-l)-cells. Diabetologia 1999; 42(7): 856–64
Xu G, Stoffers DA, Habener JF, et al. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999; 48(12): 2270–6
Stoffers DA, Kieffer TJ, Hussain MA, et al. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes 2000; 49(5): 741–8
Zhou J, Wang X, Pineyro MA, et al. Glucagon-like peptide 1 and exendin-4 convert pancreatic AR42J cells into glucagon-and insulin-producing cells. Diabetes 1999; 48(12): 2358–66
Perfetti R, Zhou J, Doyle ME, et al. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology 2000; 141(12): 4600–5
Trümper A, Trümper KTH, Arnold R, et al. Protein kinase B activation by glucose-dependent insulinotropic polypeptide and growth hormone in β-(INS-1)-cells [abstract]. Diabetologia 2000; 43Suppl. 1: A136
Creutzfeldt WO, Kleine N, Willms B, et al. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7-36) amide in type I diabetic patients. Diabetes Care 1996; 19(6): 580–6
Hvidberg A, Nielsen MT, Hilsted J, et al. Effect of glucagon-like peptide-1 (pro-glucagon 78-107amide) on hepatic glucose production in healthy man. Metabolism 1994; 43(1): 104–8
Wettergren A, Schjoldager B, Mortensen PE, et al. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci 1993; 38(4): 665–73
Nauck MA, Niedereichholz U, Ettler R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol 1997; 273 (5 Pt 1): E981–8
Young A, Denaro M. Roles of amylin in diabetes and in regulation of nutrient load [editorial]. Nutrition 1998; 14(6): 524–7
Flint A, Raben A, Astrup A, et al. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998; 101(3): 515–20
Naslund E, Barkeling B, King N, et al. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord 1999; 23(3): 304–11
Gutzwiller JP, Drewe J, Goke B, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol 1999;276 (5 Pt 2): R1541–4
Rachman J, Barrow BA, Levy JC, et al. Near-normalisation of diurnal glucose concentrations by continuous administration of glucagon-like peptide-1 (GLP-1) in subjects with NIDDM. Diabetologia 1997; 40(2): 205–11
Larsen J, Hylleberg B, Ng K, et al. Glucagon-like peptide-1 infusion must be maintained for 24 h/day to obtain acceptable glycemia in type 2 diabetic patients who are poorly controlled on sulphonylurea treatment. Diabetes Care 2001; 24(8): 1416–21
Krarup T, Saurbrey N, Moody AJ, et al. Effect of porcine gastric inhibitory polypeptide on beta-cell function in type I and type II diabetes mellitus. Metabolism 1987; 36(7): 677–82
Gutniak M, Orskov C, Holst JJ, et al. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med 1992; 326(20): 1316–22
Vilsboll T, Toft-Nielsen MB, Krarup T, et al. Evaluation of beta-cell secretory capacity using glucagon-like peptide 1. Diabetes Care 2000; 23(6): 807–12
Vilsboll T, Krarup T, Madsbad S, et al. The pathogenesis of type 2 diabetes may involve a defective second phase insulin response to GIP [abstract]. Diabetes 2001; 50Suppl. 2: A11
Hücking K, Meier J, Holst JJ, et al. Reduced otropic effect of gastric inhibitory polypeptide (GIP) in first-degree relatives of type 2 diabetic patients [abstract]. Diabetes 2001; 49Suppl. 1: A227
Kubota A, Yamada Y, Hayami T, et al. Identification of two missense mutations in the GIP receptor gene: a functional study and association analysis with NIDDM: no evidence of association with Japanese NIDDM subjects. Diabetes 1996; 45(12): 1701–5
Almind K, Ambye L, Urhammer SA, et al. Discovery of amino acid variants in the human glucose-dependent insulinotropic polypeptide (GIP) receptor: the impact on the pancreatic beta cell responses and functional expression studies in Chinese hamster fibroblast cells. Diabetologia 1998; 41(10): 1194–8
Lynn FC, Pamir N, Ng EH, et al. Defective glucose-dependent insulinotropic polypeptide receptor expression in diabetic fatty Zucker rats. Diabetes 2001; 50(5): 1004–11
Meneilly GS, Ryan AS, Minaker KL, et al. The effect of age and glycemic level on the response of the beta-cell to glucose-dependent insulinotropic polypeptide and peripheral tissue sensitivity to endogenously released insulin. J Clin Endocrinol Metab 1998; 83(8): 2925–32
Marks V. GIP: the obesity hormone. In: James WPT, Parker SW, editors. Current approaches: obesity. Southampton: Duphar Medical Relations, 1988: 13–9
Yip RG, Wolfe MM. GIP biology and fat metabolism. Life Sci 2000; 66(2): 91–103
Elliott RM, Morgan LM, Tredger JA, et al. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol 1993; 138(1): 159–66
Yip RG, Boylan MO, Kieffer TJ, et al. Functional GIP receptors are present on adipocytes. Endocrinology 1998; 139(9): 4004–7
Wasada T, McCorkle K, Harris V, et al. Effect of gastric inhibitory polypeptide on plasma levels of chylomicron triglycerides in dogs. J Clin Invest 1981; 68(4): 1106–7
Ebert R, Nauck M, Creutzfeldt W. Effect of exogenous or endogenous gastric inhibitory polypeptide (GIP) on plasma triglyceride responses in rats. Horm Metab Res 1991; 23(11): 517–21
Starich GH, Bar RS, Mazzaferri EL. GIP increases insulin receptor affinity and cellular sensitivity in adipocytes. Am J Physiol 1985; 249 (6 Pt 1): E603–7
Beck B, Max JP. Gastric inhibitory polypeptide enhancement of the insulin effect on fatty acid incorporation into adipose tissue in the rat. Regul Pept 1983; 7(1): 3–8
Oben J, Morgan L, Fletcher J, et al. Effect of the entero-pancreatic hormones, gastric inhibitory polypeptide and glucagon-like polypeptide-1(7-36) amide, on fatty acid synthesis in expiants of rat adipose tissue. J Endocrinol 1991; 130(2): 267–72
Baba AS, Harper JM, Buttery PJ. Effects of gastric inhibitory polypeptide, somatostatin and epidermal growth factor on lipogenesis in ovine adipose explants. Comp Biochem Physiol B Biochem Mol Biol 2000; 127(2): 173–82
Knapper JM, Puddicombe SM, Morgan LM, et al. Investigations into the actions of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (7-36)amide on lipoprotein lipase activity in expiants of rat adipose tissue. J Nutr 1995; 125(2): 183–8
Dawson JM, Greathead HM, Sessions VA, et al. Effect of gastric inhibitory polypeptide on bovine fat metabolism. Comp Biochem Physiol B Biochem Mol Biol 1999; 123(1): 79–88
Ranganath LR, Beety JM, Morgan LM. Inhibition of insulin, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) secretion by octreotide has no effect on post-heparin plasma lipoprotein lipase activity. Horm Metab Res 1999; 31(4): 262–6
Miyawaki K, Yamada Y, Jomori T, et al. Inhibition of GIP prevents obesity. Diabetes 2001; 50Suppl. 2: A83–4
Acknowledgements
There are no potential conflicts of interest. The author’s work was supported by grants from The Danish Medical Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holst, J.J. Gastric Inhibitory Polypeptide Analogues. BioDrugs 16, 175–181 (2002). https://doi.org/10.2165/00063030-200216030-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200216030-00002