Abstract
Background and objective: Postherpetic neuralgia (PHN) and diabetic polyneuropathy (DPN) are two common causes of peripheral neuropathic pain. Typical localized symptoms can include burning sensations or intermittent shooting or stabbing pains with or without allodynia. Evidence-based treatment guidelines recommend the 5% lidocaine (lignocaine) medicated plaster or pregabalin as first-line therapy for relief of peripheral neuropathic pain. This study aimed to compare 5% lidocaine medicated plaster treatment with pregabalin in patients with PHN and patients with DPN.
Methods: The study was a two-stage, adaptive, randomized, controlled, open-label, multicentre trial that incorporated a drug wash-out phase of up to 2 weeks prior to the start of the comparative phase. At the end of the enrolment phase, patients who fulfilled the eligibility criteria were randomized to either 5% lidocaine medicated plaster or pregabalin treatment and entered the 4-week comparative phase. The interim analysis represents the first stage of the two-stage adaptive trial design and was planned to include data from the comparative phase for the first 150 randomized patients of the 300 total planned for the trial. Patients aged ≥18 years with PHN or DPN were recruited from 53 investigational centres in 14 European countries. For this interim analysis, 55 patients with PHN and 91 with DPN (full-analysis set [FAS]), randomly assigned to the treatment groups, were available for analysis. Topical 5% lidocaine medicated plaster treatment was administered by patients to the area of most painful skin. A maximum of three or four plasters were applied for up to 12 hours within each 24-hour period in patients with PHN or DPN, respectively. Pregabalin capsules were administered orally, twice daily. The dose was titrated to effect: all patients received 150mg/day in the first week and 300 mg/day in the second week of treatment. After 1week at 300 mg/day, the dose of pregabalin was further increased to 600 mg/day in patients with high pain intensity scores. The pre-planned primary study endpoint was the rate of treatment responders, defined as completing patients experiencing a reduction from baseline of ≥2 points or an absolute value of ≥4 points on the 11-item numerical rating scale of recalled average pain intensity over the last 3 days (NRS-3), after 4 weeks of treatment. Secondary endpoints included ≥30% and ≥50% reductions in NRS-3 scores, changes in neuropathic pain symptom inventory (NPSI) scores and allodynia severity ratings.
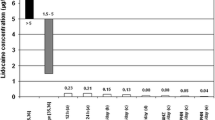
Results: Overall, 65.3% of patients treated with the 5% lidocaine medicated plaster and 62.0% receiving pregabalin responded to treatment with respect to the primary endpoint. A higher proportion of PHN patients responded to plaster treatment compared with pregabalin (63.0% vs 37.5%), whereas in the larger DPN group treatments were comparable. Both treatments improved NPSI scores and reduced allodynia severity. Patients administering lidocaine plaster experienced fewer drug-related adverse events (3.9% vs 39.2%) and there were substantially fewer discontinuations due to drug-related adverse events (1.3% vs 20.3%).
Conclusion: After 4 weeks, 5% lidocaine medicated plaster treatment was associated with similar levels of analgesia in patients with PHN or DPN but substantially fewer frequent adverse events than pregabalin.







Similar content being viewed by others
References
Cruccu G, Anand P, Attal N, et al. EFNS guidelines on neuropathic pain assessment. Eur J Neurol 2004 Mar; 11(3): 153–62
Belgrade MJ. Following the clues to neuropathic pain: distribution and other leads reveal the cause and the treatment approach. Postgrad Med 1999 Nov; 106(6): 127–32: 35-40
Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007; 132: 237–51
Volpi A, Gross G, Hercogova J, et al. Current management of herpes zoster: the European view. Am J Clin Dermatol 2005; 6(5): 317–25
Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med 2008; 9(6): 660–774
Finnerup NB, Otto M, Jensen TS, et al. An evidence-based algorithm for the treatment of neuropathic pain. Med Gen Med 2007; 9(2): 36
Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain 2005 Dec 5; 118(3): 289–305
Pfizer Limited. Lyrica capsules SPC [online]. Available from URL: http://http://emc.medicines.org.uk/emc/assets/c/html/DisplayDoc.asp?DocumentID=14651 [Accessed 2009 Jan 16]
Dworkin RH, Corbin AE, Young Jr JP, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology 2003 Apr 22; 60(8): 1274–83
Sabatowski R, Galvez R, Cherry DA, et al. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain 2004 May; 109(1–2): 26–35
van Seventer R, Feister HA, Young Jr JP, et al. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin 2006 Feb; 22(2): 375–84
Lesser H, Sharma U, LaMoreaux L, et al. Pregabalin relieves symptoms of painful diabetic neuropathy. Neurology 2004; 63: 2104–10
Richter RW, Portenoy R, Sharma U, et al. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain 2005; 6: 253–60
Rosenstock J, Tuchman M, LaMoreaux L, et al. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain 2004 Aug; 110(3): 628–38
Galer BS, Rowbotham MC, Perander J, et al. Topical lidocaine patch relieves postherpetic neuralgia more effectively than a vehicle topical patch: results of an enriched enrollment study. Pain 1999 Apr; 80(3): 533–8
Khaliq W, Alam S, Puri N. Topical lidocaine for the treatment of postherpetic neuralgia. Cochrane Database Syst Rev 2007 (2): CD004846
Rowbotham MC, Davies PS, Verkempinck C, et al. Lidocaine patch: double-blind controlled study of a new treatment method for post-herpetic neuralgia. Pain 1996 Apr; 65(1): 39–44
Argoff CE, Galer BS, Jensen MP, et al. Effectiveness of the lidocaine patch 5% on pain qualities in three chronic pain states: assessment with the Neuropathic Pain Scale. Curr Med Res Opin 2004; 20Suppl. 2: S21–8
Herrmann DN, Barbano RL, Hart-Gouleau S, et al. An open-label study of the lidocaine patch 5% in painful idiopathic sensory polyneuropathy. Pain Med 2005; 6: 379–84
Barbano RL, Herrmann DN, Hart-Gouleau S, et al. Effectiveness, tolerability, and impact on quality of life of the 5% lidocaine patch in diabetic polyneuropathy. Arch Neurol 2004 Jun; 61(6): 914–8
Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005 Jan; 113(1–2): 9–19
Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology 2003 Apr 22; 60(8): 1284–9
Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain 2004 Apr; 108(3): 248–57
Bauer P, Köhne K. Evaluation of experiments with adaptive interim analyses. Biometrics 1994; 50(4): 1029–41
Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985 Apr–Jun; 4(2): 213–26
Cochran W. Some methods for strengthening the common X2 tests. Biometrics 1954; 10: 31
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959 Apr; 22(4): 719–48
Meier T, Wasner G, Faust M, et al. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain 2003 Nov; 106(1–2): 151–8
Galer BS, Jensen MP, Ma T, et al. The lidocaine patch 5% effectively treats all neuropathic pain qualities: results of a randomized, double-blind, vehicle-controlled, 3-week efficacy study with use of the neuropathic pain scale. Clin J Pain 2002; 18(5): 297–301
Baron R, Binder A, Boesl I, et al. Long-term efficacy, safety and quality of life with lidocaine 5% medicated plaster in postherpetic neuralgia [abstract no. 787]. Pain in Europe V, 5th Congress of the European Federation of IASP chapters (EFIC); 2006 Sep 13–16; Istanbul
Hans G, Baron R, Binder A, et al. Long-term efficacy and tolerability of a 5% lidocaine-medicated plaster for the topical treatment of post-herpetic neuralgia: results of a long-term study [abstract no. 2894]. 12th World Congress on Pain; 2008 Aug 17–22; Glasgow
Glasser SP, Salas M, Delzell E. Importance and challenges of studying marketed drugs: what is a phase IV study? Common clinical research designs, registries, and self-reporting systems. J Clin Pharmacol 2007 Sep; 47(9): 1074–86
Acknowledgements
The authors would like to thank the clinical investigators who took part in this study.
Ralf Baron has received honoraria from Allergan, Schwarz, Pfizer, Grünenthal, Sanofi-Pasteur and Genzyme and has received research funding from Pfizer, Grünenthal and Genzyme. Andreas Binder has received honoraria from Allergan, Schwarz, Pfizer and Grünenthal. Victor Mayoral has received honoraria from Grünenthal. Göran Leijon has no conflicts of interest that are relevant to the content of this study. Ilona Steigerwald is an employee of Grünenthal. Michael Serpell has received honoraria from Grünenthal, and his institution received remuneration from Grünenthal to cover the costs of patient visits and recruitment during the study.
Medical writing assistance was provided by Wolters Kluwer Health and was funded by Grünenthal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baron, R., Mayoral, V., Leijon, G. et al. Efficacy and Safety of 5% Lidocaine (Lignocaine) Medicated Plaster in Comparison with Pregabalin in Patients with Postherpetic Neuralgia and Diabetic Polyneuropathy. Clin. Drug Investig. 29, 231–241 (2009). https://doi.org/10.2165/00044011-200929040-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200929040-00002