Abstract
Introduction
Peripheral neuropathic pain (PNP) is difficult to treat. Several oral drugs are recommended as first-line treatments. Nevertheless, many patients cannot obtain sufficient pain relief or do not tolerate systemically active treatments. Topical treatments, with a lower risk of systemic side effects such as lidocaine 700 mg medicated plaster, are also recommended in treatment guidelines. This analysis compares the benefit–risk balance of topical 700 mg lidocaine medicated plaster with the benefit–risk balance of oral pregabalin administration for the treatment of PNP following current recommendations on benefit–risk assessment (BRA) methodology.
Methods
The Benefit–Risk Action Team (BRAT) framework was used as structured approach. Selection of key benefits and risks was supported by a patient survey. Published randomized controlled clinical trials were the main source to identify data related to key benefits and risks. The outcome of randomized clinical trials was compared with real-world evidence (RWE) data for consistency.
Results
Identified key benefits were pain reduction and improvement in quality of life. Key risks identified were application site reactions, dizziness, confusion, weight gain, peripheral edema, and blurred vision. Overall, there was similarity in key benefits between the comparators; however, a clear advantage regarding key risks in favor of lidocaine 700 mg medicated plaster was observed. This observation was consistent across data from a direct comparison trial, randomized placebo-controlled trials, as well as data from RWE studies. The low number of randomized controlled trials for lidocaine 700 mg medicated plaster was the main limitation.
Conclusion
Guided by the opinion of patients regarding key benefits and risks deemed important for treatments of peripheral neuropathic pain, our analysis showed that lidocaine 700 mg medicated plaster has a more favorable benefit–risk balance compared to pregabalin (300 and 600 mg daily).
Similar content being viewed by others
Why carry out this study? |
The limited efficacy of available treatments for peripheral neuropathic pain, their specific side effect profiles, and the lack of clinical studies that would help to guide the optimal sequence of therapy in a given patient, highlight the need for a systematic comparison of the benefit–risk balance of various treatments in this indication. |
To the author’s knowledge, this is the first benefit–risk analysis following a structured framework approach (BRAT) comparing the benefit–risk balances of a topical and an oral treatment option recommended for the treatment of peripheral neuropathic pain (lidocaine 700 mg medicated plaster and pregabalin). |
Peripheral neuropathic pain is a prevalent disease with a high need for effective and well-tolerated treatments. |
Several drugs are recommended as first- or second-line treatments in current guidelines for peripheral neuropathic pain, including several classes of systemically active medications, as well as topical medications such as the lidocaine 700 mg medicated plaster. |
This work aimed to compare the benefit–risk balance of topical lidocaine 700 mg medicated plaster to the benefit–risk balance of oral pregabalin administration (300 and 600 mg) for the treatment of peripheral neuropathic pain following current recommendations for benefit–risk assessment and incorporating the patients’ perspective. |
What was learned from the study? |
The benefit–risk profile of lidocaine 700 mg medicated plaster is more favorable compared to the benefit–risk profile of pregabalin 300 mg and 600 mg for the treatment of peripheral neuropathic pain. |
The conclusion from this benefit–risk assessment supports the recommendation of the lidocaine 700 mg medicated plaster in current treatment guidelines for peripheral neuropathic pain. |
Direct comparison in clinical trials between oral and topical treatments is complex because of blinding of the administration and potential unblinding due to differential benefit–risk profiles. Our approach shows that alternative ways of evaluating benefit–risk may be feasible, adding to the knowledge on treatment alternatives. |
Introduction
A best estimate of the prevalence of pain with neuropathic characteristics in the general population is likely to lie between 6.9% and 10% [1]. Further research [2] showed that the prevalence of predominantly peripheral neuropathic pain (PNP) amongst patients with neuropathic pain was 12.9% (95% CI 1.5; 14.3). Neuropathic pain remains difficult to treat. The typical approach to the management of a patient with neuropathic pain is to initiate treatment with conservative (i.e., pharmacological and complementary) therapies before interventional strategies, such as nerve blocks and neuromodulation, are used [3]. Several oral drugs are recommended as first-line treatments in (not necessarily consistently across all) European and international treatment guidelines [4,5,6], including pregabalin and gabapentin (originally developed as antiepileptic medications), tricyclic antidepressants (TCA), selective serotonin reuptake inhibitors (SSRI), and serotonin–noradrenalin reuptake inhibitors (SNRI). Despite availability of these treatment options, many patients cannot obtain sufficient pain relief or do not tolerate adequate doses of systemic drug therapies because of side effects [7]. Lidocaine 700 mg medicated plaster, a topical treatment option associated with low systemic exposure and a low risk for systemic side effects, is also recommended as first- or second-line treatment option for neuropathic pain in some clinical guidelines [4,5,6].
The limited efficacy of available treatments, their specific side effect profile, and the lack of clinical studies that would help to guide the physician in the optimal sequence of therapy in a given patient, highlight the need for a systematic comparison of the benefit–risk balance of available treatment options.
In the past, assessments of the relationship between benefits and risks of medical interventions were often not conducted in a systematical and transparent way, and therefore subjective and prone to inconsistency in interpretation [8].
Hence several initiatives were taken in the last decade both in the USA and European Union (EU) [9, 10], leading to comprehensive guidelines on benefit–risk assessment (BRA) [10, 11]. These guidelines recommend the use of qualitative frameworks and, in case of an unclear outcome, follow-up with quantitative methods to provide all necessary elements for a transparent and objective benefit–risk trade-off. Frameworks commonly used and recommended include BRAT (Benefit–Risk Action Team) and PrOACT-URL [10]. Common elements of these frameworks are stepwise approaches to BRA assisting in selecting, organizing, summarizing, and communicating evidence relevant to benefit–risk decisions. Useful visualizations comprise value trees to map all benefits and risks that are being considered in a hierarchical diagram as well as effects tables enabling the display of outcome data for all selected benefits and risks in one place.
The patients’ perspective is becoming increasingly important in decision-making [12, 13], and can be integrated in BRAs to elicit the patients’ choice in terms of the most important benefits and risks.
In this article, we compare the benefits and risks of the topical lidocaine 700 mg medicated plaster to those of oral administration of pregabalin 300 mg and 600 mg for the treatment of PNP according to the structured, qualitative BRAT framework, supplemented by a patient survey designed to select the key benefits and risks from a patient perspective.
To our knowledge, this is the first publication of a BRA following a structured framework approach comparing a topical with a systemically active treatment option mentioned in current treatment guidelines for PNP.
Methods
BRAT Framework and BRA Team Composition
The BRAT framework, proposed by the Pharmaceutical Research and Manufacturers of America (PhRMA), is a structured and stepwise approach to BRA assisting in selecting, organizing, summarizing, and communicating evidence relevant to benefit–risk decisions [14]. Following the available guidance [10] on BRA and applying the BRAT framework, this evaluation was conducted by an internal team composed of a clinical expert, drug safety expert, and biostatistician (all employees of Grünenthal GmbH) with support of an experienced external consultant.
Selection of Comparator for This BRA
Although several oral treatments are recommended in treatment guidelines for PNP, only one has been selected as comparator for topical lidocaine 700 mg medicated plaster for this BRA, in order to reduce complexity and to facilitate clear communication of the outcome.
The selection of pregabalin as comparator for lidocaine 700 mg medicated plaster was based on the following considerations:
-
1.
The selected comparator should have the lowest possible number of warnings and contraindications amongst the recommended first-line treatments, so that the benefit–risk balance would not be differentially impacted by certain risks in specific subpopulations.
A review of the Summaries of Product Characteristics (SmPCs) of recommended first-line oral treatments (gabapentinoids, TCAs, SSRIs, SNRIs) for risks and contraindications was done. Other oral treatments were generally associated with more contraindications/warnings and potentially severe side effects in certain patient groups as well as an apparent higher potential to cause drug–drug interactions than the gabapentinoids pregabalin and gabapentin (SmPCs accessed from eMC webpage).
-
2.
There should be comprehensive data available from clinical trials for the preferred comparator.
Pregabalin and gabapentin have a broad indication in neuropathic pain in the EU. Therefore, availability of comprehensive data from clinical trials across subtypes/underlying etiologies of peripheral neuropathic pain was expected.
Pregabalin but not gabapentin was preferred for this BRA given the more extensive clinical trial data available for pregabalin based on recent Cochrane reviews [15, 16].
-
3.
There should be a publicly available risk management plan (RMP) as a sound starting point for selection of the key risks.
An RMP approved by centralized procedure was only found for pregabalin but not for gabapentin on the European Medicines Agency (EMA) webpage.
-
4.
The comparator preferred on the basis of the criteria above should not obviously be inferior in efficacy in the treatment of neuropathic pain in comparison to other recommended first-line treatments.
An evaluation was done for randomized trials directly comparing efficacy of pregabalin in peripheral neuropathic pain conditions with at least one other recommended first-line treatment. A search for publications was done in Embase (August 2019) with the search strategy (“neuropathic pain” /exp OR “neuropathic pain”) AND pregabalin AND [randomized controlled trial]/lim. Titles and abstracts were screened to identify respective trials. Overall, 262 records were retrieved, of which nine trials matched the scope. In five trials, pregabalin was compared to duloxetine [17,18,19,20,21], in four trials to amitriptyline [21,22,23,24], in two trials to gabapentin [23, 25], and in one trial to venlaflaxine [18]. Indications across the trials were diabetic peripheral polyneuropathy (DPN) (six trials), chemotherapy-induced neuropathic pain (one trial), neuropathic cancer pain (one trial), and pain due to peripheral nerve injury (one trial). None of the other recommended first-line treatments were superior to pregabalin in efficacy in any of the trials.
Overall, based on the criteria listed above, selection of pregabalin as comparator for this BRA appeared appropriate.
Dose Selection
For pregabalin, a dose–response curve for efficacy in neuropathic pain, but also a dose-dependent increase in frequency of adverse events has been shown [26]. Both 300 mg and 600 mg daily doses (mid-range and maximum of recommended doses per pregabalin SmPC, respectively) were selected as separate comparators for this BRA to account for potential differences in the benefit to risk balance of different doses.
For the lidocaine 700 mg medicated plaster, abstraction of the number of plasters was done (within the recommended number of 1–3 plasters) given the localized effect and assuming that adequate adjustment of the number of plasters has been done in clinical trials from which data was included.
Selection of Key Benefits and Risks and Associated Outcome Measures
An initial broad set of benefits and risks was identified, which was further reduced to the final set of those effects that matter most for the benefit–risk trade-off. Value trees (Figs. 1, 2, 3) show the outcome of this “pruning” process. The initial set of benefits and risks (initial value tree, Fig. 1) and the set of benefits and risks given in the pruned value tree (Fig. 2) were generated on the basis of medico-scientific discussions within our expert group. A dedicated patient survey was conducted afterwards and considered for the final set of benefits and risks as shown in the final value tree (Fig. 3). The selection process was guided by the principle that benefits and risks should apply to the broad PNP population (i.e., not only to a small subset) and that the selected outcomes should be sufficiently distinct (to avoid double counting) and commonly used in clinical trials (to ensure availability of sufficient data for the evaluation).
Key Benefits
The following outcomes were selected as key benefits: pain reduction and improvement in quality of life. Pain reduction is usually reported in clinical trials as “change in numerical rating scale (NRS) or verbal rating scale (VRS) pain scores from baseline” or as “endpoint values corrected for baseline”, and “responder rate of at least 30% reduction in pain compared to baseline”. As these two outcome measures provide sufficiently distinct and complementary information, they were both retained. For the analyses VRS pain scores were transformed into NRS scores by simple calculation. Change in quality of life is commonly assessed in clinical trials by the EQ5D (a standardized measure of health-related quality of life developed by the EuroQol Group) and the Patient Global Impression of Change (PGIC). In order to make a comparison based on a larger number of trials, both (EQ5D and PGIC) were selected.
On the basis of the patient survey (supplemental material 3 and 4), “rapid reduction of pain/ fast onset of action” and “sustaining control of pain” were also considered to be important benefits; however, they were not retained as oral administration of pregabalin requires titration to effect not compatible with rapid onset of action, and sustained pain control could lead to double counting given that the reduction of pain was measured over at least 4 weeks in trials that were included.
Key Risks
The RMPs current when this evaluation was initiated [27, 28] were used as starting point for the selection of key risks. The important identified risks contained in the RMP list of safety concerns (Table 1) are expected to have a major impact on patients and/or public health. Classification as an important identified risk also typically requires sufficient evidence for a causal association of the product with the risk. The RMP of lidocaine 700 mg medicated plaster only contained one safety concern (local application site reactions) which was retained for display in the effects tables. The Pregabalin Pfizer RMP list of safety concerns contained nine important identified risks. This list was further reduced to four risks for which data were extracted and displayed in effects tables: “weight gain”, “peripheral edema”, “CNS effects (increasing the risk of accidental injury)”, “vision-related events”.
Risks that were finally disregarded for the final effects within this BRA are summarized in Table 2.
For each of the retained key risks, specific adverse event (AE) terms, as extracted from literature and available in the source data extraction table, were assigned. Specific considerations for this step are described in supplemental material 6.
Patient Survey
General Setup
To provide directional input from patients on the important benefits and risks from their perspective, a small patient survey was conducted by an independent agency. The survey also included questions to physicians prescribing medications to treat neuropathic pain, which is not further described here, as it was out of scope of this BRA. This survey was conducted in accordance with the European Pharmaceutical Market Research Association (EphMRA) and Data Protection legislation. All patients provided written consent for participation in this survey. Consistent with the conduct of surveys focusing on patients’ attitudes without impact on treatment nor requirement to use medication, an approval of an ethical review board was not required.
The patient part of this online survey included a sample of 25 patients between 35 and 75 years from Germany, Italy, Spain, and Ireland with a diagnosis of painful peripheral diabetic polyneuropathy (pDPN), postherpetic neuropathy (PHN), or postsurgical neuropathic pain (psNP) of at least 3 months duration, and who were treated with either pregabalin or lidocaine 700 mg medicated plaster at the time of the survey. The recruitment was performed mainly via pain specialists, who selected eligible patients on the basis of the inclusion criteria, except for Ireland where a patient panel (patient self-assessment for inclusion criteria) was used. To control for potential time effects regarding frequency and intensity of pregabalin side effects, patients with different therapy durations were selected. Thus, at the time of the survey five patients had been taking pregabalin for 2–4 weeks, 10 patients for 5 weeks to 3 months, and two patients for more than 3 months. Among the patients on lidocaine 700 mg medicated plaster, three patients had been using the plasters for up to 8 weeks, and five patients for 7 to a maximum of 36 months at the time of the survey.
Questions to Elicit the Patients’ Preference Regarding Key Benefits and Risks
Participants were asked to select the three most important benefits the medication should provide. Patients could choose from a list containing a broad selection of benefits including those of the initial value tree (see Fig. 1 for the initial value tree; supplementary material 3 for all 11 items of the list patients could choose from). Patients were also asked to indicate the three side effects with the strongest negative impact on the willingness to use the medication. For this purpose, patients were instructed to choose from a list of 21 possible side effects those with the strongest negative impact on willingness to use the medication, and in addition those considered to be unacceptable in light of the previously selected three most important benefits. This list included the initial broad selection of side effects (Fig. 1, initial value tree), and side effects of other pain medications (for the full list, see supplementary material 4). In addition, patients could also mention other side effects that they would regard as important.
The results of the patient survey are provided in supplemental materials 3 and 4.
Impact of Results on Selection of Key Benefits and Risks
On the basis of the outcome of the patient survey, the only change made was replacement of the AE term “somnolence” with “confusion”. The latter was rated as more important by the patients and determines together with the term “dizziness” the risk for “CNS effects (increasing the risk of accidental injury)”.
Data Relating to Outcome Measures
Sources of Data Relating to Outcome Parameters
The primary source of data related to outcome parameters for key favorable and unfavorable effects was published literature from trials with pregabalin and/or lidocaine 700 mg medicated plaster in PNP of localized origin. As a basis for further processing within this BRA, data from published trials (until Nov 2018) identified by an external group (Kleijnen Systematic Reviews Ltd, York, UK) in the context of a recent systematic literature review [29] was used.
Main Inclusion Criteria for Randomized Controlled Clinical Trials
Randomized controlled clinical trials were only considered for this BRA if the duration of treatment was at least 4 weeks (a shorter period of pain relief was not considered of high importance in the light of the chronicity of the condition under investigation, and that pregabalin is typically carefully titrated to a targeted dose in order to minimize side effects), and if a minimum of 20 patients had been treated with pregabalin 300 and/or 600 mg, or lidocaine 700 mg medicated plaster in the respective trials. Indications included pDPN, PHN, and psNP as common PNP conditions.
Inclusion of Real-World Evidence Studies
In order to provide a holistic view on benefits and risks, data from RWE studies close to clinical practice have been considered for the overall discussion. As a result of their different and diverse design, these data were, however, not integrated into the effects tables for randomized controlled clinical trials. Data on key favorable/unfavorable effects from RWE studies were captured also when not completely matching the rigorous selection criteria required for entry into the effects tables displaying data from the randomized controlled clinical trials.
Handling of Potential Data Bias
AE Reporting Cutoff
Publications of trials often only report adverse event data above certain frequency thresholds (often 2–5%). Therefore, data from Grünenthal’s clinical trial database was used where applicable (i.e., more granular data available than from publications of respective clinical trials). This applies for the direct comparison trial of lidocaine 700 mg medicated plaster vs. pregabalin 600 mg [30]. In addition, the weighted average attributable AE risks calculated for pregabalin for this BRA from published studies were compared with the attributable risks for the same AEs from a pooled analysis of randomized clinical trials [26]. Attributable AE risks were closely matching in this comparison, suggesting that AE reporting cutoffs did not introduce a substantial bias. Therefore, AE data from publications were considered adequate and kept for this BRA.
Placebo Effect of Oral Placebo vs. Topical Placebo Plaster
A limitation in comparing data from placebo-controlled trials of lidocaine 700 mg medicated plaster with data from placebo-controlled trials of oral administration of pregabalin lies within a potential different effect of oral placebo compared to topical placebo plasters. While high placebo responses with various oral treatments in trials evaluating the treatment effect on pain in various neuropathic pain conditions [31] and similarly with transdermal systems [32] have been reported, the available data do not allow to conclude on the differential placebo effect of oral versus transdermal placebo. No correction or sensitivity analysis was done in this BRA for potentially different placebo effects of oral placebo versus topical placebo plasters.
Placebo Response Over Time
A different placebo response over time may constitute a confounder when differences vs. placebo are compared from trials of different duration. Noteworthy, a sustained placebo effect with strongest effects in the first 4 weeks with smaller further reductions of pain leading to a plateau in the treatment advantage over placebo at weeks 4 to 12 has consistently been shown across neuropathic pain trials [31]. This is in line with the observations of placebo-controlled trials with pregabalin in peripheral neuropathic pain [33]. For this BRA the duration of included randomized trials ranges generally from 4 to 12 weeks treatment duration. Considering that a plateau in the treatment advantage over placebo has been shown in neuropathic pain trials within this time frame, no substantial bias based on different durations of trials included in this BRA is expected at least for the key effect of pain reduction. No correction or further sensitivity analysis was done in this BRA to address a potential bias in difference to placebo based on trial duration.
Visualizations: Value Trees and Effects Tables
So-called value trees are recommended BRA tools to enhance visualization of the “pruning” process, i.e., the process of selecting the key effects that matter most for the benefit–risk trade-off. In our work, three value trees were generated. The initial value tree (Fig. 1) shows a broad list of effects (without assignment of specific outcome measures) initially discussed in the BRA expert team. A “pruned value tree” shows the effects and outcome measures that remained after further medico-scientific discussions within the BRA team (Fig. 2). The final value tree (Fig. 3) shows the effects and outcomes after the patient survey outcomes had been considered. For these final key effects and outcomes, data from randomized controlled clinical trials was gathered into effects tables.
The source data for this BRA contained trials of different design, i.e., one open-label direct comparison trial, several randomized placebo-controlled trials for either the lidocaine 700 mg medicated plaster or pregabalin 300 mg/600 mg, and non-randomized RWE studies. As a result of the different designs of these trials, one effects table for the direct comparison trial was generated (supplementary material 1), as well as one effects table integrating the effects of placebo-controlled trials of either lidocaine 700 mg medicated plaster or pregabalin (supplementary material 2) into weighted average relative risks/differences, and weighted average attributable risks. For RWE studies, data on key benefits and risks were collected separately per trial, without calculation of average effects in the effects tables.
Results
Direct Comparison Trial
Lidocaine 700 mg medicated plaster and pregabalin 600 mg were directly compared in an open-label randomized trial in a population of patients with PHN and pDPN [30]. The primary objective of this trial was to test lidocaine 700 mg medicated plaster for non-inferiority against pregabalin (titrated to effect) in a 4-week comparative phase. The effects table containing data from the comparative phase of the trial displays all selected key favorable and unfavorable effects (supplementary material 1). With respect to key benefits (pain reduction and quality of life), the results from this trial are very similar for both comparators. There is a numerical trend in favor of lidocaine 700 mg medicated plaster, which is more pronounced in the population with PHN than pDPN.
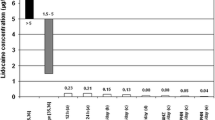
The differences in AE rates observed in this trial between lidocaine 700 mg medicated plaster and pregabalin are as follows: subjects in the pregabalin 600 mg treatment group reported “weight increase” (4 of 153 = 2.6%), “peripheral edema” (6 of 153 = 3.9%), “confusion” (4 of 153 = 2.6%), “dizziness” (18 of 153 = 11.8%), and “vision blurred” (3 of 153 = 2.0%), while in the lidocaine 700 mg medicated plaster treatment group one subject (of 155 = 0.7%) reported peripheral edema, and seven subjects (of 155 = 4.5%) reported application site reactions.
Randomized Trials Comparing Either Lidocaine 700 mg Medicated Plaster or Pregabalin 300 mg and/or 600 mg to Placebo
Supplementary material 2 shows the effects table for placebo-controlled trials of either lidocaine 700 mg medicated plaster or pregabalin (300 mg and 600 mg daily separately presented).
Some trials pertaining to lidocaine 700 mg medicated plaster as well as pregabalin 300 mg and 600 mg used “change from baseline” calculation for mean pain reduction from baseline, while others used “endpoint corrected for baseline”. These results cannot be combined and are therefore displayed separately in the effects table. Forest plots showing the mean differences to placebo for all included trials can be found in supplemental material 7.
Key Benefits, Pain Reduction, Difference in Mean Pain Reduction (Measured on 11-Point NRS Scale) from Baseline vs. Placebo
Comparing weighted average differences of the studies reporting “endpoint corrected for baseline” only, there is a slight numerical advantage of pregabalin 300 mg (− 0.90; eight trials) and pregabalin 600 mg (− 1.00; 10 trials) over the lidocaine 700 mg medicated plaster (− 0.24; two trials). In contrast, for studies using “change from baseline calculation”, there is a clear advantage for the lidocaine 700 mg medicated plaster (− 4.30; one trial) over pregabalin 300 mg (− 0.20; four trials) and pregabalin 600 mg (− 0.35; two trials) with the limitation that there is only one study [34] for the lidocaine 700 mg medicated plaster in this latter category.
The variability of observed differences in mean pain reduction vs. placebo across the trials included was high.
Key Benefits, Pain Reduction, Difference in Responder Rate of ≥ 30% Pain Reduction from Baseline vs. Placebo
The weighted average relative risk in responder rate of at least 30% pain reduction from baseline vs. placebo was slightly higher for pregabalin 300 mg (1.53; seven trials) and for pregabalin 600 mg (1.61; eight trials) as compared to the lidocaine 700 mg medicated plaster (1.22; one trial).
The variability for the difference in mean pain reduction from baseline vs. placebo in trials included for pregabalin was high. Only one trial [35] could be included for lidocaine 700 mg medicated plaster.
Key Benefits, Quality of Life, Change in EQ5D Score from Baseline vs. Placebo
For lidocaine 700 mg medicated plaster, no difference to placebo (relative change vs. placebo 0.00; one trial) was shown [35].
Similarly, no difference to placebo was found for pregabalin 300 mg in two [36, 37] trials (both trials report “change from baseline”); one further trial [38] showed a mean change vs. placebo (“endpoint corrected for baseline”) of 0.08 for pregabalin 300 mg resulting in an overall weighted average relative difference of 0.00 (for “change from baseline”; two trials) and 0.08 (“endpoint corrected for baseline”; one trial).
For pregabalin 600 mg differences vs. placebo of 0.01 (“change from baseline”; one trial [39]) and respectively 0.03 and 0.14 were reported (“endpoint corrected for baseline” [38, 40]), for an average mean relative difference of 0.08 to placebo.
Key Benefits, Improvement in Quality of Life, Rate of PGIC “At Least Minimally Improved” vs. Placebo
The weighted average relative risk for this outcome showed a numerical advantage in favor of the lidocaine 700 mg medicated plaster (4.79; two trials) compared to pregabalin 300 mg (1.30; four trials) and pregabalin 600 mg (1.45; five trials). There was, however, a substantial difference in the relative risk vs. placebo observed (1.08 [35] and 27.00 [34]) in the two trials included for the lidocaine 700 mg medicated plaster. Also, for pregabalin 300 mg and 600 mg, there was considerable variability in the observed relative risks vs. placebo across the trials included.
The weighted attributable risk (0.17; two trials) was similar for the lidocaine 700 mg medicated plaster and pregabalin 600 mg (five trails) and slightly in favor of the lidocaine 700 mg medicated plaster as compared to pregabalin 300 mg (0.15; four trials).
Key Risks
There was one placebo-controlled trial [35] included for the evaluation of AEs of lidocaine 700 mg medicated plaster. The data used for this analysis are from Grünenthal clinical trial database (i.e., without cutoff for frequency). The literature search identified additional publications of placebo-controlled randomized clinical trials of lidocaine 700 mg medicated plaster; however, these publications did not contain any AE codes relating to the risks selected for this BRA.
For pregabalin 300 and 600 mg, the number of trials included differs between the key unfavorable effects as values can only be provided from trials in which the respective AEs were reported. In some publications certain AEs may not have been reported because they were either not observed or a frequency cutoff for AE reporting was used.
Also for some specific AE categories for pregabalin 300 mg and 600 mg the number of underlying trials to obtain the weighted average relative effect and attributable risk may be different, as it is not possible to calculate a weighted effect for trials containing zero values for certain effects for placebo. Average weighted relative risks for subjects in the pregabalin 300 mg and 600 mg vs. placebo groups are clearly higher than for the lidocaine 700 mg medicated plaster vs. placebo for “weight increase”, “peripheral edema”, “confusion”, “dizziness”, and “vision blurred”, whereas the average weighted relative risk for patients under lidocaine 700 mg medicated plaster treatment vs. placebo for local application site reactions is higher than for pregabalin (300 mg and 600 mg) vs. placebo. The weighted average attributable risks for pregabalin 300 mg and 600 mg were 0.07/0.07 for “weight gain” (9/10 trials), 0.05/0.09 for “peripheral edema” (14/11 trials), 0.02/0.05 for “confusion” (4/4 trials), 0.12/0.20 for “dizziness” (14/13 trials), and 0.02/0.04 for “vision blurred”/ “amblyopia” (8/7 trials). These data indicate a higher risk for all effects apart from weight gain for pregabalin 600 mg as compared to pregabalin 300 mg.
RWE Studies
Overall, 19 published studies suitable for inclusion could be found. A table of key favorable/unfavorable effects as well as study design, main inclusion criteria, study duration, and indication are provided in supplemental material 5. In 11 out of these 19 studies, lidocaine 700 mg medicated plaster (in the publications referred to as lidocaine 5% plaster) was the treatment investigated, nine studies examined effects of pregabalin, one publication [41] included both pregabalin and lidocaine 5% plaster. Indications reported for lidocaine 700 mg medicated plaster were PHN, pDPN, psNP, and cancer-related neuropathic pain (crNP). There were eight prospective studies (two of which were open-label extensions of previous studies), and three retrospective studies based on medical records or patient databases. Treatment duration in these studies ranged from 3 weeks up to 48 months. Indications in published studies for pregabalin included pDPN, PHN, HIV-related NP, chemotherapy-induced NP, crNP, back pain with neuropathic component, and mixed neuropathic pain indications of not further specified origin. Six of these studies had a prospective design including two open-label extension phases of previous trials, and two studies were based on retrospective review of medical records/patient databases. Treatment duration ranged from 4 to 52 weeks. Pregabalin doses were 150 mg to 600 mg in most of the studies.
Key Benefits, “Pain Reduction”, Mean Pain Reduction
Six publications for the lidocaine 700 mg medicated plaster contained data on mean pain reduction [42,43,44,45,46,47]. The values for mean pain reduction on a 11-point NRS or VRS scale in these studies ranged from − 2.0 to − 3.4.
Also, publications of six studies for pregabalin [48,49,50,51,52,53] reported on mean pain reduction ranging from − 2.5 to − 3.8 on an 11-point NRS/VRS scale.
Key Benefits, “Pain Reduction”, 30% Responder Rate in Pain Reduction
Two publications for the lidocaine 700 mg medicated plaster [42, 46] report the percentage of patients with at least 30% pain reduction: in a prospective open-label study of 3-week treatment duration in the indication pDPN [42] a responder rate of 70% has been shown; in a 12-week prospective trial in postsurgical/post-traumatic neuropathic pain [46] a 65% responder rate for at least 30% pain reduction was reported. A total of 60.8% of patients experienced at least 30% reduction in pain intensity in a prospective open-label study examining effects of a 4-week treatment with pregabalin [48]. After 6 weeks of treatment with pregabalin in an open-label observational study, 89% of patients with pDPN, 84% of patients with back pain, and 85% of patients with crNP had at least 30% pain reduction [53].
Key Benefit “Improvement in Quality of Life” (EQ5D and PGIC)
Limited data from published RWE studies with the lidocaine 700 mg medicated plaster or pregabalin on change in EQ5D or PGIC were found. An improvement in EQ5D VAS score (continuous variable) of 26.4 was reported in a prospective open-label study [50] of 8-week duration with pregabalin (indications mainly pDPN, PHN, and 11% in other neuropathies). In another prospective, open-label study [51] it was reported that 87% of patients treated with pregabalin for pDPN, PHN, HIV-related peripheral NP, and chemotherapy-induced NP had at least much improvement in PGIC. A total of 49.8% of patients had at least much improvement in PGIC in a prospective open-label 4-week study with pregabalin in pDPN/PHN and 81% had at least minimal improvement [48]. For the lidocaine 700 mg medicated plaster, it was shown that more than 70% of patients had at least much improved PGIC rating throughout measurement timepoints during the 12-month initial trial [47] and the extension study up to 48 months [54].
Key Unfavorable Effects
Considering the general limitations of a less rigorously controlled design than for randomized clinical trials, AE data from RWE studies are consistent with data from the randomized clinical trials as presented in this work.
Lidocaine 700 mg Medicated Plaster
Application site reactions were the most frequently reported adverse events (frequencies range from 0 to 15%) in most studies with the lidocaine 700 mg medicated plaster. None of the other key unfavorable effects selected for this BRA were identified in any of the publications for lidocaine 700 mg medicated plaster with one exception. During a 4-year period (3-year follow-up open extension study including a previous 12-month trial) in a predominantly elderly population (mean age 71.3 ± 9.2 years), seven cases of dizziness were reported [54].
Pregabalin
In most studies with pregabalin, dizziness, edema, and weight gain are reported, albeit with a broad range of frequencies (dizziness 0.9–20.7%; edema 0.6–15.4%; weight gain 8–22%). Confusion and amblyopia, which are known to occur with lower incidence rates [26], have only been reported by one and two publications, respectively [55, 56]. These AEs might either not have been captured in most studies or their frequency was below the reporting threshold of most publications.
Discussion
Most of the currently recommended pharmaceutical treatment options for PNP are oral medications including pregabalin and gabapentin, TCAs, SSRIs, and SNRIs [4,5,6]; however, these drugs have reportedly overall limited efficacy and are associated with systemic side effects [7]. Guidelines are also recommending topical treatments such as lidocaine 700 mg medicated plaster [4,5,6] for the treatment of PNP.
To the authors knowledge, this is the first BRA following a structured framework approach as recommended in current BRA guidance to compare the benefit–risk balances of a topical and an oral treatment in the indication PNP.
Published randomized controlled clinical trials supplemented by data from the Grünenthal clinical trial database for lidocaine 700 mg medicated plaster, when data from literature did not provide sufficient granularity for the analysis, were the primary source of data relating to key benefits and risks. This BRA not only considered published literature on randomized controlled clinical trials but in addition included an assessment of RWE data to provide a holistic view considering also data close to clinical practice.
Oral administration of pregabalin (300 mg and 600 mg) has been selected as comparator to topical lidocaine 700 mg medicated plaster for this BRA. On the basis of a comparison of the safety profiles and head-to-head studies, pregabalin does not appear to have a benefit–risk balance inferior to other recommended first-line treatments. In addition, as a sound starting point for the selection of key risks, comprehensive data from clinical trials and an RMP summary (part of a centralized registration procedure in EU) could be used for pregabalin.
The selection of the key benefits and key risks has been done by an internal expert panel at Grünenthal (consisting of clinical expert, drug safety expert, biostatistics expert) supported by an external consultant. Objective medico-scientific criteria were used for pruning of key effects consistent with current recommendations on BRA. Input from the patient perspective on the perception of importance of key benefits and risks has been obtained by a patient survey and has been considered in the final selection of key effects.
The results of this analysis show a more favorable benefit–risk balance for lidocaine 700 mg medicated plaster than for pregabalin (300 mg and 600 mg) in the treatment of PNP: available data indicate large similarity between the two drugs in terms of the key benefits (pain reduction and improvement in quality of life); however, there is only one key risk attributable to the lidocaine 700 mg medicated plaster (“local application site reactions”), as compared to several key risks for pregabalin (“dizziness” and “confusion”, “weight gain”, “peripheral edema”, “visual impairment”). Moreover, the patient survey revealed that “local application site reactions” have a lower impact on acceptability of a medication to treat PNP than each of the included key risks pertaining to the safety profile of pregabalin.
Similarity in the key favorable effects consistently applies for the trial directly comparing the lidocaine 700 mg medicated plaster with pregabalin 600 mg as well as for integrated effects of placebo-controlled trials. Also, RWE studies suggest that lidocaine 700 mg medicated plaster and pregabalin 300 mg to 600 mg are effective in reducing pain to a similar extent in “real-life use”.
The outcome of this BRA following a structured framework is in line with the recent investigation of the comparative benefit–risk balance of these two medications by a systematic literature review and network meta-analysis [29], and support the recommendation of lidocaine 700 mg medicated plaster in recent treatment guidelines despite the limited number of placebo-controlled randomized clinical trials available for this treatment option.
The low number of placebo-controlled clinical trials with lidocaine 700 mg medicated plaster is considered the main limitation of this BRA. Another limitation consists of considerable variability in efficacy outcomes for both comparators, which limits firm conclusions on the true magnitude of effects.
A potential confounding factor may lie in the comparison of differences vs. placebo from trials of different duration, based on a different placebo response over time. However, a sustained placebo effect leading to a plateau in the treatment advantage over placebo across neuropathic pain trials at weeks 4 to 12 has been shown [31], consistent with the observations of placebo-controlled trials with pregabalin in PNP [33]. Since for this BRA the duration of included randomized trials ranges generally from 4 to 12 weeks treatment duration, no substantial bias based on different durations of included trials is expected at least for the key effect of pain reduction.
Another limitation in comparing data from placebo-controlled trials of lidocaine 700 mg medicated plaster with those of the oral pregabalin treatment lies within a potential different effect of oral placebo preparations compared to topical placebo plasters. Direct comparison in clinical trials between oral and topical treatments is complex. While blinding is desired in such trials, this might be hampered by potential unblinding due to the differential benefit–risk profile of oral and topical treatments. No firm conclusions can be drawn on the exact impact of potentially different placebo effects of oral placebo vs. topically applied placebo plasters until well-designed trials to examine this will have been performed.
Acknowledging the limitations, our approach shows that alternative ways of evaluating benefits and risks may be feasible and add to the knowledge on treatment alternatives.
Conclusions
This structured framework (BRAT) approach that included the patients’ perspective on benefits and risks confirms the more favorable benefit–risk balance of lidocaine 700 mg medicated plaster compared to pregabalin (300 mg and 600 mg daily) in the treatment of PNP.
References
van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–62. https://doi.org/10.1016/j.pain.2013.11.013.
Failde I, Dueñas M, Ribera MV, et al. Prevalence of central and peripheral neuropathic pain in patients attending pain clinics in Spain: factors related to intensity of pain and quality of life [published correction appears in J Pain Res. 2018 Oct 17;11:2397]. J Pain Res. 2018;11:1835–47. https://doi.org/10.2147/JPR.S159729.
Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nature Reviews Disease Primers. London: Nature Publishing Group; 2017; Juhaeri J. Benefit–risk evaluation: the past, present and future. Ther Adv Drug Safety. January 2019.
Schlereth T, et al. Diagnose und nicht interventionelle Therapie neuropathischer Schmerzen, S2k-Leitlinie, 2019. In: Deutsche Gesellschaft für Neurologie (Hrsg.), Leitlinien für Diagnostik und Therapie in der Neurologie. www.dgn.org/leitlinien Accessed 19 Sep 2019.
Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157:1599–606.
Moisset X, Bouhassira D, Avez Couturier J, et al. Pharmacological and non-pharmacological treatments for neuropathic pain: systematic review and French recommendations. Rev Neurol (Paris). 2020;176(5):325–52. https://doi.org/10.1016/j.neurol.2020.01.361.
Attal N, Bouhassira D. Pharmacotherapy of neuropathic pain: which drugs, which treatment algorithms? Pain. 2015;156:S104–14.
Juhaeri J. Benefit-risk evaluation: the past, present and future. Ther Adv Drug Saf. 2019;26(10):2042098619871180. https://doi.org/10.1177/2042098619871180.PMID:31489173;PMCID:PMC6712756.
Coplan PM, Noel RA, Levitan BS, et al. Development of a framework for enhancing the transparency, reproducibility and communication of the benefit-risk balance of medicines. Clin Pharmacol Ther. 2011;89:312–5.
PROTECT Consortium. Recommendations for the methodology and visualisation techniques to be used in the assessment of benefit and risk of medicines; 2013. http://www.imi-protect.eu/documents/HughesetalRecommendationsforthemethodologyandvisualisationtechniquestobeusedintheassessmento.pdf. Accessed 30 Apr 2018.
Hughes D, Waddingham E, Mt-Isa S, et al. Recommendations for benefit-risk assessment methodologies and visual representations. Pharmacoepidemiol Drug Saf. 2016;25:251–62.
European Medicines Agency. Patients and consumers. www.ema.europa.eu/en/partners-networks/patients-consumers. Accessed Jul 2021.
US Food and Drug Administration. Patient preference information–voluntary submission, review in premarket approval applications, humanitarian device exemption applications, and De Novo requests, and inclusion in decision summaries and device labeling. Silver Spring, MD: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, 2016.
Noel R, Hermann R, Levitan B, Watson D, van Goor K. Application of the benefit-risk action team (BRAT) framework in pharmaceutical R&D: results from a pilot program. Drug Inf J. 2012;46:736–43.
Derry S, Bell R, Straube S, Wiffen PJ, Aldington D, Moore R. Pregabalin for neuropathic pain in adults. Cochrane Database of Syst Rev. 2019;1:7076. https://doi.org/10.1002/14651858.CD007076.pub3.
Wiffen PJ, Derry S, Bell RF, Rice ASC, Tölle T, Phillips T, Moore R. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6:7938. https://doi.org/10.1002/14651858.CD007938.pub4.
Joarchi K, Memari M, Azargashb E, Saadat N. Efficacy and safety of duloxetine and pregabalin in Iranian patients with diabetic peripheral neuropathic pain: a double-blind, randomized clinical trial. J Diab Metab Disord. 2019;18(2):575–82.
Kardanpou N, Khorvash F, Khorvash F, Memarzadeh M. A comparative trial of duloxetine hydrochloride, venlaflaxine hydrochloride, and pregabalin on the sensory symptoms in patients wit dibetic polyneuropathy. J Isfahan Med School. 2018;35(462):1885–91.
Enomoto H, Yasuda H, Nishiyori A, et al. Duloxetine in patients with diabetic peripheral neuropathic pain in Japan: a randomized, double-blind, noninferiority comparative trial with pregabalin. J Pain Res. 2018;11:1857–68.
Avan R, Janbabaei G, Hendouei N, et al. The effect of pregabalin and duloxetine treatment on quality of life of breast cancer patients with taxane-induced sensory neuropathy: a randomized clinical trial. J Res Med Sci. 2018;23:7.
Boyle J, Eriksson MEV, Gribble L, et al. Randomized, placebo-controlled comparion of amitriptyline, duloxetine, and pregabaline in patients with chronic diabetic peripheral neuropathic pain: Impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care. 2012;35(12):2451–8.
Arshad I, Zulfiqar H, Shafi B. To compare the efficacy of pregabalin and amitryptiline for pain relief in patients with diabetic peripheral nuropathy. J Pioneer Med Sci. 2018;8(2):21–5.
Mishra S, Bhatnagar S, Goyal GN, Rana SPS, Upadhya SP. A comparative efficacy of amitryptiline, gabapentin, and pregabalin in neuropathic cancer pain: a prospective randomized double-blind placebo-controlled trial. Am J Hosp Palliat Med. 2012;29(3):177–82.
Shabbir B, Shafi F, Mahboob F. Amitriptyline vs pregabalin in painful diabetic neuropathy a randomised placebo-based trial. Pak J Med Health Sci. 2011;5(4):745–7. https://www.pjmhsonline.com/2011/oct_dec/pdf/ZZK%20Amitriptyline%20Vs%20Pregabalin%20in%20Painful%20Diabetic%20Neuropathy.pdf.
Kelle B, Yavuz F, Yasar E, Goktepe AS. The efficacy of gabapentin and pregabalin in the treatmet of neuopathic pain due to peripheral nerve injury. J Musculoskelet Pain. 2012;20(4):300–5.
EMA. EPAR Lyrica, scientific discussion. 2004. https://www.ema.europa.eu/en/documents/scientific-discussion/lyrica-epar-scientific-discussion_en.pdf. Accessed 3 May 2019.
EMA/247834/2014 Summary of the Risk Management Plan (RMP) for pregabalin Pfizer (pregabalin). 2014. https://www.ema.europa.eu/en/documents/rmp-summary/pregabalin-pfizer-epar-risk-management-plan-summary_en.pdf. Accessed 20 Mar 2019.
Risk Management Plan lidocaine 700 mg medicated plaster version 5.3, signature date 21 Nov 2016.
Buksnys T, Armstrong N, Worthy G, et al. Systematic review and network meta-analysis of the efficacy and safety of lidocaine 700 mg medicated plaster vs pregabalin. Curr Med Res Opin. 2020;36(1):101–15. https://doi.org/10.1080/03007995.2019.1662687.
Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. Efficacy and safety of 5% lidocaine (lignocaine) medicated plaster in comparison with pregabalin in patients with postherpetic neuralgia and diabetic polyneuropathy: interim analysis from an open-label, two-stage adaptive, randomized, controlled trial. Clin Drug Investig. 2009;29(4):231–41.
Tuttle AH, Tohyama S, Ramsay T, et al. Increasing placebo responses over time in US clinical trials of neuropathic pain. Pain. 2015;156(12):2616–26.
Ondarza A, Lewis F, Womack T. Placebo effects of transdermal NSAIDs. Applied Clinical Trials Online. https://www.appliedclinicaltrialsonline.com/view/placebo-effect-transdermal-nsaids. Accessed 26 May 2020.
Freeman R, Emir B, Parsons B. Predictors of placebo response in peripheral neuropathic pain: insights from pregabalin clinical trials. J Pain Res. 2015;8:257–68.
Sansone P, Passavanti MB, Fiorelli A, et al. Efficacy of the topical 5% lidocaine medicated plaster in the treatment of chronic post-thoracotomy neuropathic pain. Pain Manag. 2017;7(3):189–96. https://doi.org/10.2217/pmt-2016-0060.
Palladini M, Boesl I, Koenig S, Buchheister B, Attal N. Lidocaine medicated plaster, an additional potential treatment option for localized post-surgical neuropathic pain: efficacy and safety results of a randomized, placebo-controlled trial. Curr Med Res Opin. 2019;35:1–1.
Eli Lilly and Company. A trial for treatment of pain in patients with diabetic neuropathy. NCT00785577. ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2010. https://ClinicalTrials.gov/show/NCT00785577. Accessed 18 Dec 2018.
Ziegler D, Duan WR, An G, Thomas JW, Nothaft W. A randomized double-blind, placebo-, and active-controlled trial of T-type calcium channel blocker ABT-639 in patients with diabetic peripheral neuropathic pain. Pain. 2015;156(10):2013–20.
Tolle T, Freynhagen R, Versavel M, Trostmann U, Young JP Jr. Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind trial. Eur J Pain. 2008;12(2):203–13.
Markman J, Resnick M, Greenberg S, et al. Efficacy of pregabalin in post-traumatic peripheral neuropathic pain: a randomized, double-blind, placebo-controlled phase 3 trial. J Neurol. 2018;265:2815–24.
Moon DE, Lee DI, Lee SC, et al. Efficacy and tolerability of pregabalin using a flexible, optimized dose schedule in Korean patients with peripheral neuropathic pain: a 10-week, randomized, double-blind, placebo-controlled, multicenter trial. Clin Ther. 2010;32(14):2370–85.
Katz P, Pegoraro V, Liedgens H. Characteristics, resource utilization and safety profile of patients prescribed with neuropathic pain treatments: a real-world evidence study on general practices in Europe—the role of the lidocaine 5% medicated plaster. Curr Med Res Opin. 2017;33(8):1481–9. https://doi.org/10.1080/03007995.2017.1335191.
Barbano RL, Herrmann DN, Hart-Gouleau S, Pennella-Vaughan J, Lodewick PA, Dworkin RH. Effectiveness, tolerability, and impact on quality of life of the 5% lidocaine patch in diabetic polyneuropathy. Arch Neurol. 2004;61(6):914–8. https://doi.org/10.1001/archneur.61.6.914.
Brabant S, Nagels W. Topical lidocaine 5% patch for the treatment of chronic postsurgical neuropathic skin pain [Abstract PA62]. Pain Pract. 2009;9(S1):35.
Correa-Illanes G, Calderón W, Roa R, Piñeros JL, Dote J, Medina D. Treatment of localized post-traumatic neuropathic pain in scars with 5% lidocaine medicated plaster. Local Reg Anesth. 2010;3:77–83. https://doi.org/10.2147/LRA.S13082.
Garzón-Rodríguez C, Casals Merchan M, Calsina-Berna A, López-Rómboli E, Porta-Sales J. Lidocaine 5 % patches as an effective short-term co-analgesic in cancer pain. Preliminary results. Support Care Cancer. 2013;21(11):3153–8. https://doi.org/10.1007/s00520-013-1948-7.
Hans G, Joukes E, Verhulst J, Vercauteren M. Management of neuropathic pain after surgical and non-surgical trauma with lidocaine 5% patches: study of 40 consecutive cases. Curr Med Res Opin. 2009;25(11):2737–43.
Hans G, Sabatowski R, Binder A, Boesl I, Rogers P, Baron R. Efficacy and tolerability of a 5% lidocaine medicated plaster for the topical treatment of post-herpetic neuralgia: results of a long-term study. Curr Med Res Opin. 2009;25(5):1295–305.
Baron R, Brunnmüller U, Brasser M, May M, Binder A. Efficacy and safety of pregabalin in patients with diabetic peripheral neuropathy or postherpetic neuralgia: open-label, non-comparative, flexible-dose study. Eur J Pain. 2008;12(7):850–8. https://doi.org/10.1016/j.ejpain.2007.12.004.
Ogawa S, Suzuki M, Arakawa A, Yoshiyama T, Suzuki M. Long-term efficacy and safety of pregabalin in patients with postherpetic neuralgia: results of a 52-week, open-label, flexible-dose study. Masui. 2010;59(8):961–70.
Perez-Lloret S, Rojas GM, Menoni MC, et al. Pregabalin beneficial effects on sleep quality or health-related quality of life are poorly correlated with reduction on pain intensity after an 8-week treatment course. Clin Neuropharmacol. 2012;35(1):21–4.
Pfizer. Pregabalin treatment of peripheral neuropathic pain associated with diabetic peripheral NeP (DPN), postherpetic neuralgia (PHN), HIV-related NeP (HIV), and chemotherapy induced NeP. NCT00407511. ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2008. https://ClinicalTrials.gov/show/NCT00407511. Accessed 28 Nov 2018.
Satoh J, Yagihashi S, Baba M, et al. Efficacy and safety of pregabalin for treating neuropathic pain associated with diabetic peripheral neuropathy: a 14 week, randomized, double-blind, placebo-controlled trial. Diabet Med. 2011;28(1):109–16.
Toelle TR, Varvara R, Nimour M, Emir B, Brasser M. Pregabalin in neuropathic pain related to DPN, cancer and back pain: analysis of a 6-week observational study. Open Pain J 2012;5(1):1-11.
Sabatowski R, Hans G, Tacken I, Kapanadze S, Buchheister B, Baron R. Safety and efficacy outcomes of long-term treatment up to 4 years with 5% lidocaine medicated plaster in patients with post-herpetic neuralgia. Curr Med Res Opin. 2012;28(8):1337–46.
Usta C, Akbas M. Pregabalin induced adverse drug effects in the treatment of peripheral neuropathic pain. Klinik Psikofarmakol Bulteni. 2011;21(3):219–24.
Mittal M, Pasnoor M, Mummaneni RB, et al. Retrospective chart review of duloxetine and pregabalin in the treatment of painful neuropathy. Int J Neurosci. 2011;121(9):521–7.
Acknowledgements
Funding
This study was funded by Grünenthal GmbH including the Rapid Service Fee of the journal Pain and Therapy.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Authors’ Contributions
Conception, design: Ingo Sabatschus, Irmgard Bösl, Marlou Prevoo, Michael Forstner. Statistical analysis: Marlou Prevoo. Data interpretation and drafting of the manuscript: Ingo Sabatschus, Mariëlle Eerdekens, Irmgard Bösl, Arne Sprünken, Oliver Galm, Marlou Prevoo, Michael Forstner.
Disclosures
Ingo Sabatschus, Irmgard Bösl, Marlou Prevoo, Mariëlle Eerdekens, Arne Sprünken and Oliver Galm are employees of Grünenthal GmbH. Michael Forstner is owner and managing director of Mesama-Consulting.
Compliance with Ethics Guidelines
All patients gave their consent to participate in the patient survey that was part of the overall methodology. This survey was conducted in accordance with the European Pharmaceutical Market Research Association (EphMRA) and Data Protection legislation. Consistent with the conduct of surveys focusing on patients’ attitudes without impact on treatment nor requirement to use medication, an approval of an ethical review board was not required. Apart from the patient survey, this article aggregates data of previously conducted and published studies also not requiring ethic review board review.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sabatschus, I., Bösl, I., Prevoo, M. et al. Comparative Benefit–Risk Assessment for Lidocaine 700 mg Medicated Plaster and Pregabalin in Peripheral Neuropathic Pain Following a Structured Framework Approach. Pain Ther 11, 73–91 (2022). https://doi.org/10.1007/s40122-021-00340-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-021-00340-2