Abstract
Introduction: Different oral sustained-release (SR) formulations of tramadol have been introduced in pain treatment in order to prolong the dosage interval to improve convenience for the patient. The objective of this study was to compare tramadol pharmacokinetics and intra- and intersubject variability after replicate single-dose administrations of a multiple-units SR formulation (capsule) and a single-unit formulation (tablet).
Methods: This was a randomised, single-dose, single-centre study with an open-label, four-period, two-sequence, two-formulation, replicate crossover design in healthy subjects under fed conditions. The main outcome measures were the intra- and intersubject variance of the area under the concentration-time curve from 0 to 12 hours (AUC12) and maximum concentration (Cmax), as well as the mean AUC12 and CPmax for tramadol. Study drugs were a tramadol SR multiple-units formulation (capsule) and a tramadol SR single-unit formulation (tablet), each containing tramadol hydrochloride 100mg. The time interval from 0 to 12 hours of AUC12 of the single-dose design corresponds to the recommended twice-daily dosage interval for both study drugs during long-term treatment.
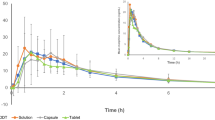
Results: The two formulations were equivalent in the area under the curve (AUC∞: 2411 vs 2527 μg · h/L). However, capsules led to a lower Cmax (148.6 vs 183.2 μg/L), to a later time to reach Cmax (5.9 vs 4.9 hours), and to a longer half-value duration (13.4 vs 10.4 hours). In addition, intrasubject variability of AUC12 was significantly smaller for capsules than for tablets (p = 0.041). Capsules also produced smaller intra- and intersubject variability in plasma concentrations during the first 2.5 and 3.0 hours after administration, respectively (p < 0.05).
Conclusion: Although tramadol SR capsules and tramadol SR tablets led to an equivalent systemic exposure to the drug, capsules provided a smoother and more extended plasma profile. In addition, in the case of capsules, bioavailability was subjected to lower variability in terms of both rate and extent of absorption.







Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Scott L, Perry C. Tramadol: a review of its use in perioperative pain. Drugs 2000 Jul; 60(1): 139–76
Raffa RB, Nayak RK, Lia S, et al. The mechanism(s) of action and pharmacokinetics of tramadol hydrochloride. Rev Contemp Pharmacother 1995; 6: 485–97
Bamigbade TA, Langford RM. The clinical use of tramadol hydrochloride. Pain Rev 1998; 5: 155–82
Shipton E. Tramadol: present and future. Anaesth Intensive Care 2000 Aug; 28(4): 363–74
Lintz W, Barth H, Osterloh G, et al. Bioavailability of enteral tramadol formulations: 1st communication: capsules. Arzneimittel Forschung 1986; 36 (II) 8: 1278–83
Raber M, Schulz HU, Schürer M, et al. Pharmacokinetic properties of tramadol sustained release capsules: 2nd communication: investigation on relative availability and food interaction. Arzneimittel Forschung 1999; 49 (II) 7: 588–93
Raber M, Hoffman S, Junge K, et al. Analgesic efficacy and tolerability of tramadol 100mg sustained-release capsules in patients with moderate to severe chronic low back pain. Clin Drug Invest 1999 Jun; 17(6): 415–23
Sorge J, Stadler T. Comparison of the analgesic efficacy and tolerability of tramadol 100mg sustained-release tablets and tramadol 50mg capsules for the treatment of chronic low back pain. Clin Drug Invest 1997 Sep; 14(3): 157–64
Hummel T, Roscher S, Pauli E, et al. Assessment of analgesia in man: tramadol controlled release vs tramadol standard formulation. Eur J Clin Pharmacol 1996; 51: 31–8
Benchgaard H, Nielsen GH. Controlled-release multiple-unit and single unit doses: a literature review. Drug Dev Ind Pharm 1978; 4(1): 53–67
Guidance for industry. Statistical approaches to establishing bioequivalence. US Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER), 2001 Jan, Rockville, MD
Chow SC, Liu JP. Design and analysis of bioavailability and bioequivalence studies. New York: Marcel Dekker Inc, 1992: 253–88
Holm S. A simple sequentially rejective multiple test procedure. Scan J Statist 1979; 6: 65–70
Note for guidance on modified release oral and transdermal dosage forms: section II (pharmacokinetic and clinical evaluation), EAMEA. CPMP/EWP/280/96. London: 1999 Jul 28
Comparison of the release rate profiles of tramadol SR capsules with tramadol SR tablets. Company internal report; 2004 Jul (Data on file, VIATRIS)
Petrone D, Kamin M, Olson W. Slowing the titration rate of tramadol HCl reduces the incidence of discontinuation due to nausea and/or vomiting: a double-blind randomized trial. J Clin Pharm Ther 1999; 24: 115–23
Ruoff G. Slowing the initial titration rate of tramadol improves tolerability. Pharmacotherapy 1999; 19(1): 88–93
Bechgaard H, Benzon A. Critical factors influencing gastrointestinal absorption: what is the role of pellets? Acta Pharm Technol 1982; 28(2): 149–56
Fara JW. Physiological limitations: gastric emptying and transit of dosage forms. In: Prescott LF, Nimmo WS, editors. Rate control in drug therapy. Edinburgh: Churchill Livingstone, 1985: 144–50
Davis SS, Hardy JG, Fara JW. Transit of pharmaceutical dosage forms through the small intestine. Gut 1986; 27: 886–92
Acknowledgements
The principal investigator of this study was Katharina Erb, MD, Frankfurt am Main, Germany. The study was supported by ASTA Medica AG, Frankfurt am Main, Germany (predecessor of VIATRIS GmbH & Co. KG, Bad Homburg, Germany).
The authors thank Dr Diana A. Taylor, Medical Information, VIATRIS GmbH & Co. KG, for editing this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cnota, P.J., Nowak, H., Tagarro, I. et al. Tramadol SR Formulations. Clin. Drug Investig. 25, 435–443 (2005). https://doi.org/10.2165/00044011-200525070-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200525070-00002