Abstract
Objective
To compare the systemic bioavailability of two ibuprofen formulations, Pedea® (ibuprofen intravenous [IV] formulation) and Imbun® (ibuprofen intramuscular [IM] formulation) in 18 healthy male volunteers.
Methods
Each subject received a 5 mg/kg dose of ibuprofen base as a short 15-minute IV infusion as the Pedea® ibuprofen IV formulation or as the reference Imbun® IM formulation. Concentrations of R- and S-ibuprofen were measured by a validated HPLC method with a lower limit of quantification of 0.100 μg/mL.
Results
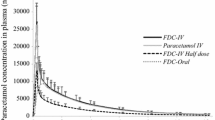
A single 5 mg/kg injection of Pedea® was well tolerated. The most frequent adverse event was a mild to moderate burning sensation along the injection vein probably related to the study treatments. The maximum serum concentration (Cmax) of R- and S- ibuprofen ranged from 20 to 35 μg/mL with both formulations. No statistical differences were observed for either Cmax or area under the plasma concentration-time curve (AUC). 90% CIs calculated for Cmax and AUC from time zero to infinity (AUC∞) of R-ibuprofen and S-ibuprofen were included in the bioequivalence range 0.80–1.25. Based on AUC∞, the mean (SD) relative bioavailability of Pedea® (ibuprofen IV formulation) versus the Imbun® IM reference formulation was 1.06 (0.17) for R-ibuprofen and 1.05 (0.08) for S-ibuprofen.
Conclusion
This study showed that Pedea® (test formulation) is bioequivalent to Imbun® IM (reference formulation) for both R-ibuprofen and S-ibuprofen. The Imbun® IM formulation (lyophilisate) could be replaced by the Pedea® ready-to-use IV solution. Moreover, these results allow a comparison of safety and efficacy data previously generated with either formulation. Consequently, neonatologists, who previously used the IM formulation intravenously for the treatment of patent ductus arteriosus in preterm infants, will now be able to administer the new ready-to-use IV solution of ibuprofen directly.






Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
VanOvermeire B, Smets K, Lecoutere D, et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med 2000; 343(10): 674–81
VanOvermeire B, Follens I, Hartmann S, et al. Treatment of patent ductus arteriosus with ibuprofen. Arch Dis Child 1997; 76: F179–84
Varvarigou A, Bardin C, Beharry K, et al. Early ibuprofen administration to prevent patent ductus arteriosus in premature newborn infants. JAMA 1996; 275(N7): 539–44
Mosca F, Bray M, Lattanzio M, et al. Comparative evaluation of the effects of indomethacin and ibuprofen on cerebral perfusion and oxygenation in preterm infants with patent ductus arteriosus. J Pediatr 1997; 131: 549–54
Patel J, Roberts I, Azzopardi D, et al. Randomized double-blind controlled trial comparing the effects of ibuprofen with indomethacin on cerebral hemodynamics in preterm infants with patent ductus arteriosus. Pediatr Res 2000; 47(1): 36–42
Pezzati J. Effects of indomethacin and ibuprofen on mesenteric and renal blood flow in preterm infants with patent ductus arteriosus. Pediatrics 1999 Dec; 135(6): 733–8
Internal CEPHAC Validation Report No. CP005315
Internal CEPHAC Method Validation SOP No. 860
Hauschke D, Steinijans VW, Diletti E. A distribution-free procedure for the statistical analysis of bioequivalence study. Int J of Clin Pharmacol Ther Tox. 1992; 28: No.2 (72–78), 1992; 30( Suppl. 1): S37–43
Davies NM. Clinical pharmacokinetics of ibuprofen, the first 30 years. Clin Pharmacokinet 1998; 34(2): 101–54
Jamali F, Singh NN, Pasutto FM, et al. Pharmacokinetics of ibuprofen enantiomers in humans following oral administration of tablets with different absorption rates. Pharm Res 1988; 5(1): 40–3
Jamali F, Mehvar R, Russel AS, et al. Human pharmacokinetics of ibuprofen enantiomers following different doses and formulations: intestinal chiral inversion. J Pharm Sci 1992; 81(3): 221–5
Oliari J, Tod M, Nicolas P, et al. Pharmacokinetics of ibuprofen enantiomers after single and repeated doses in man. Biopharm Drug Dispos 1992; 13: 337–44
Hall SD, Rudy AC, Knight PM, et al. Lack of presystemic inversion of (R) to (S)-ibuprofen in humans. Clin Pharmacol Ther 1993, 53(4): 393–400
Acknowledgements
The authors have provided no information on sources of funding or on conflicts of interest directly relevant to the content of this study.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chassard, D., Geneteau, A., Gualano, V. et al. Bioequivalence Study of Two Ibuprofen Formulations Administered Intravenously in Healthy Male Volunteers. Clin. Drug Investig. 24, 739–747 (2004). https://doi.org/10.2165/00044011-200424120-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200424120-00005