Abstract
Objective
To evaluate the cost effectiveness of subcutaneous interferon-β-1a (IFNβ-1a) 44μg three times weekly in relapsing-remitting multiple sclerosis (RRMS) using an econometric model.
Methods
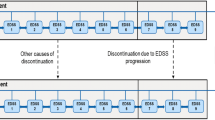
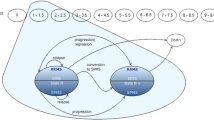
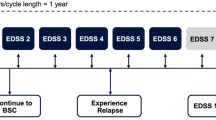
Data on RRMS patients treated with IFNβ-1a 22 or 44μg subcutaneously three times weekly or placebo for up to 4 years were obtained from the Prevention of Relapses and disability by Interferon-β-1a Subcutaneously in Multiple Sclerosis (PRISMS) study. The area under the Expanded Disability Status Scale (EDSS) score-time curve was used as a measure of disability and the effectiveness of therapy was expressed as EDSS-months of disability prevented. Costs were calculated for the UK and France, and results were projected to 10 and 20 years using a time series regression model.
Results
Over 10 years, treatment with IFNβ-1a 44μg subcutaneously three times weekly prevented 121 EDSS-months of additional disability at a cost of Euros (€)732 each (year of costing 2000). Over 20 years, 321 EDSS-months were saved at a cost of €359 per month (year of costing 2000).
Conclusion
This analysis indicated that IFNβ-1a 44μg subcutaneously three times weekly is cost effective in RRMS and that treatment becomes increasingly cost effective over time.










Similar content being viewed by others
Notes
1The use of tradenames is for product identification purposes only and does not imply endorsement.
References
PRISMS Study Group and the University of British Columbia MS/MRI Analysis Group. PRISMS-4: long-term efficacy of interferon-beta-1a in relapsing MS [published erratum Neurology 2001 Sep; 57 (6): 1146]. Neurology 2001 Jun; 56(12): 1628–36
IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis: I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993 Apr; 43(4): 655–61
Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis: the Multiple Sclerosis Collaborative Research Group (MSCRG) [published erratum Ann Neurol 1996 Sep; 40 (3): 480]. Ann Neurol 1996 Sep; 39(3): 285–94
Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind, placebo controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995 Jul; 45(7): 1268–76
National Institute for Clinical Excellence. Beta interferon and glatiramer acetate in the treatment of multiple sclerosis: final appraisal determination [online]. Available from URL: http://www.nice.org.uk [Accessed 2002 Jan 11]
Parkin D, Jacoby A, McNamee P, et al. Treatment of multiple sclerosis with interferon beta: an appraisal of cost-effectiveness and quality of life. J Neurol Neurosurg Psychiatry 2000 Feb; 68(2): 144–9
Kendrick M, Johnson KI. Long term treatment of multiple sclerosis with interferon-beta may be cost-effective. Pharmacoeconomics 2000 Jul; 18(1): 45–53
Jönsson B, Jönsson L, Kolbelt G. Modelling disease progression and the effect of treatment in secondary progressive multiple sclerosis. Stockholm: School of Economics, 1999
Stalmeier PF, Chapman GB, de Boer AG, et al. A fallacy of the multiplicative QALY model for low-quality weights in students and patients judging hypothetical health states. Int J Technol Assess Health Care 2001 Fall; 17(4): 488–96
Liu C, Li Wan Po A, Blumhardt LD. “Summary measure” statistic for assessing the outcome of treatment trials in relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 1998 Jun; 64(6): 726–9
PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis [published erratum Lancet 1999 Feb; 353 (9153): 678]. Lancet 1998 Nov; 352(9139): 1498–504
Goodin DS, Frohman EM, Garmany Jr GP, et al. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines [published erratum Neurology 2002 Aug; 59 (3): 480]. Neurology 2002 Jan; 58(2): 169–78
Murphy N, Haas J, Konig N, et al. Economic evaluation of multiple sclerosis in the UK, Germany and France. Pharmacoeconomics 1998; 13: 607–22
Dura G, Paelinck JHP, editors. Econometrics of health care. Dordrecht: Kluwer Academic Publishers, 1991
Sadovnick AD, Ebers GC, Wilson RW, et al. Life expectancy in patients attending multiple sclerosis clinics. Neurology 1992 May; 42(5): 991–4
Liu C, Blumhardt LD. Disability outcome measures in therapeutic trials of relapsing-remitting multiple sclerosis: effects of heterogeneity of disease course in placebo cohorts. J Neurol Neurosurg Psychiatry 2000 Apr; 68(4): 450–7
Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet 2001 May; 357(9268): 1576–82
Acknowledgements
Preparation of this study report was made possible by financial support from Serono International SA, Geneva, Switzerland. The authors have provided no information on conflicts of interest directly relevant to the content of the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lepen, C., Coyle, P., Vollmer, T. et al. Long-Term Cost Effectiveness of Interferon-β-1a in the Treatment of Relapsing-Remitting Multiple Sclerosis. Clin. Drug Investig. 23, 571–581 (2003). https://doi.org/10.2165/00044011-200323090-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200323090-00003