Abstract
Background: Mirtazapine is a well tolerated and effective antidepressant agent that is currently available as a tablet. Patient compliance with antidepressant treatment that has to be swallowed is very low. Therefore, an orally disintegrating formulation of mirtazapine, which dissolves in the mouth, was devised to improve patient compliance.
Objective: To establish the bioequivalence of these two formulations of mirtazapine.
Design: Under fasting conditions, participants received in a two-way, single-dose crossover fashion the orally disintegrating formulation of mirtazapine (30mg tablet) [Remeron® Soltab™] and the marketed formulation of mirtazapine (30mg tablet) [Remeron®], separated by a 2-week washout period.
Patients and Participants: 40 healthy individuals (20 men, 20 women).
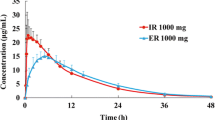
Main Outcome Measures and Results: The ratio of geometric means (new formulation: marketed formulation) of pharmacokinetic parameters was 1.072 for the peak concentration of mirtazapine (Cmax) [90% confidence interval (CI) 0.948 to 1.212], 1.103 for the area under the concentration-time curve from zero to the last time-point with a measurable concentration (AUC0-tlast) [90% CI 1.052 to 1.156] and 1.101 for the area under the concentration-time curve from zero to infinity (AUC0-∞) [90% CI 1.049 to 1.156]. The two study formulations were found to be bioequivalent, as all the 90% CIs fell within the acceptance range of 0.80 to 1.25. The adverse events reported were not unexpected and were more likely related to the protocol than to the drug. Somnolence was the most common adverse event, followed by fatigue and dizziness. No clinically significant effects on laboratory safety parameters, vital signs or ECG were observed.
Conclusion: A new orally disintegrating formulation of mirtazapine was shown to be bioequivalent to the marketed tablet formulation. This formulation may increase compliance in depressed patients and therefore offer an appropriate alternative for depressed patients currently taking tablets.



Similar content being viewed by others
References
Hirschfeld RMA. Long-term drug treatment of unipolar depression. Int Clin Psychopharmacol 1996; 11: 211–7
Kasper S. Clinical efficacy of mirtazapine: a review of metaanalyses of pooled data. Int Clin Psychopharmacol 1995; 10: 25–35
Sitsen JMA, Moors J. Mirtazapine, a novel antidepressant, in the treatment of anxiety symptoms: results from a placebocontrolled trial. Drug Invest 1994; 8: 339–44
Marttila M, Jääskeläinen J, Järvi R, et al. A double-blind study comparing the efficacy and tolerability of mirtazapine and doxepin in patients with major depression. Eur Neuro-psychopharmacol 1995; 5: 441–6
Bremner JD. A double-blind comparison of Org 3770, amitriptyline and placebo in major depression. J Clin Psychiatry 1995; 56: 519–25
Wheatley DP, van Moffaert M, Timmerman L, et al. Mirtazapine: efficacy and tolerability in comparison with fluoxetine in patients with moderate to severe major depressive disorder. J Clin Psychiatry 1998; 59: 306–12
Leinonen E, Skarstein J, Behnke K, et al. Efficacy and tolerability of mirtazapine vs citalopram: a double-blind, randomized study in patients with major depressive disorder. Clin Psychopharmacol 1999; 14: 329–37
Benkert O, Szegedi A, Kohnen R. Mirtazapine compared with paroxetine in major depression. J Clin Psychiatry 2000; 61: 656–63
Kasper S, Praschak-Reider N, Tauscher J, et al. A risk-benefit assessment of mirtazapine in the treatment of depression. Drug Saf 1997; 17: 251–64
Montgomery SA. Safety of mirtazapine: a review. Int Clin Psychopharmacol 1995; 10Suppl. 4: 37–45
Montgomery SA, Reimitz P-E, Zivkov M. Mirtazapine versus amitriptyline in the long-term treatment of depression: a double-blind placebo-controlled study. Int Clin Psychopharmacol 1998; 13: 63–73
Schutte AJ, Leinonen E, Skarstein J, et al. Mirtazapine has similar long-term efficacy and tolerability to citalopram and faster onset of action in the treatment of major depression. Int J Neuropsychopharmacol 2000; 3Suppl. 1: S193
Voortman G, Paanakker JE. Bioavailability of mirtazapine from Remeron® tablets after single and multiple oral dosing. Hum Psychopharmacol Clin Exp 1995; 10: S83–S96
Timmer CJ, Sitsen JMA, Delbressine LP. Clinical pharmacokinetics of mirtazapine. Clin Pharmacokinet 2000; 38: 461–74
Sitsen JMA, Maris FA, Timmer CJ. Concomitant use of mirtazapine and cimetidine: a drug-drug interaction study in healthy male subjects. Eur J Clin Pharmacol 2000; 56: 389–94
Sitsen JMA, Voortman G, Timmer CJ. Pharmacokinetics of mirtazapine and lithium in healthy male subjects. J Psychopharmacol 2000; 14: 172–6
Diletti E, Hauschke D, Steinjans VW. Sample size determination for bioequivalence assessment by means of confidence intervals. Int J Clin Pharmacol Ther Tox 1992; 30Suppl. 1: 551–8
Ruigt GSF, Kemp B, Groenhout CM, et al. Effect of the antidepressant Org 3770 on human sleep. Eur J Clin Pharmacol 1990; 38: 551–4
Acknowledgements
The research was performed at Farma Research BV, Nijmegen, The Netherlands, and the study was funded by Organon.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van den Heuvel, M.W., Kleijn, H.J. & Peeters, P.A.M. Bioequivalence Trial of Orally Disintegrating Mirtazapine Tablets and Conventional Oral Mirtazapine Tablets in Healthy Volunteers. Clin. Drug Investig. 21, 437–442 (2001). https://doi.org/10.2165/00044011-200121060-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200121060-00007