Summary
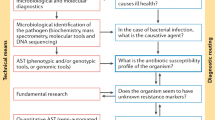
Cephalosporin drugs are stable, soluble at high concentrations and possess a characteristic ultraviolet absorption spectrum, which allows easy quantification. They are therefore relatively easy to work with under laboratory conditions. This chapter provides an overview of the laboratory tests available for assessing antimicrobial activity in research and clinical practice, and highlights the usefulness and drawbacks of such tests in the prediction of clinical efficacy with particular reference to newer cephalosporins, which are for oral administration.
Similar content being viewed by others
References
Blumberg PM, Strominger JL. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev 1974; 38: 291–335
Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Ann Rev Microbiol 1979; 33: 113–37
Georgopapadakou NH, Liu FY. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother 1980; 18: 148
Murphy TF, Barza M, Park JT. Penicillin-binding proteins in Clostridium perfringens. Antimicrob Agents Chemother 1981; 20: 809
Piddock LJV, Wise R. Properties of the penicillin-binding proteins of four species of the genus Bacteroides. Antimicrob Agents Chemother 1986; 29: 825
Tomasz A. Penicillin-binding proteins and the antibacterial effectiveness of β-lactam antibiotics. Rev Infect Dis 1986; 8 Suppl. 3: S260–S78
Waxman DJ, Strominger JL. Penicillin-binding proteins and the mechanism of action of β-lactam antibiotics. Ann Rev Biochem 1983; 52: 825–69
Donowitz GR, Mandell GL. Cephalosporins. In: Mandell GL, et al. editors. Principles and practice of infectious diseases, 3rd ed. New York: Churchill Livingstone, 1990: 246–55
Gutmann L, Vincent S, Billot-Klein D, et al. Involvement of penicillin-binding protein 2 with other penicillin-binding proteins in lysis of Escherichia coli by some β-lactam antibiotics alone and in synergistic lytic effect of amdinocillin (mecillinam). Antimicrob Agents Chemother 1986; 30: 906
Hanberger H. Pharmacodynamic effects of antibiotics. Studies on bacterial morphology, initial killing, postantibiotic effect and effective regrowth time. Scand J Infect Dis Suppl. 1992; 81: 1–52
Hamilton-Miller JMT. Microbiological investigation of cephalosporins. Drugs 1987; 34 Suppl. 2: 23–43
Mine Y, Kamimura T, Watanabe Y, et al. In vitro antibacterial activity of FK482, a new orally active cephalosporin. J Anti-biot 1988; XLI: 1873–87
Yokota T, Suzuki E, Arai K. Cefdinir, its in vitro antibacterial activity, binding affinity to bacterial PBPs, stability to β-lactamases, and synergy of bactericidal effect with serum complement and mouse cultured macrophages. [English abstract] Chemotherapy (Tokyo) 1989; 37 Suppl. 2: 29
Turcotte A, Simard M, Bergeron MG. Differential, penetration, distribution and in vivo efficacy of cefdinir (CEF), a new semi-synthetic cephalosporin in the periphery (P) and core (C) of infected fibrin clots. Proceedings of the 34th ICAAC, Orlando, Florida, 1994
Hatano K, Nishino T. Morphologic alterations of Staphylococcus aureus and Streptococcus pyogenes exposed to cefdinir, a new oral broad-spectrum cephalosporin. Chemotherapy 1994; 40: 73–79
Rosenblatt JE. Laboratory tests used to guide antimicrobial therapy. Mayo Clin Proc 1991; 66: 942–8
Barry AL, Schoenknecht FD, Shadomy S, et al. Interlaboratory variabilityof disc diffusion and agar dilution susceptibility tests with cefamandole and cephalothin. Curr Microb 1978; 1: 277
Snell JJS, Brown DEJ, Gardner PS. Comparison of results from two antibiotic susceptibility testing trials that formed part of the United Kingdom national external quality assessment scheme. J Clin Pathol 1984; 37: 321
Murray PR, Jorgensen JH. Quantitative susceptibility test methods in major United States medical centers. Antimicrob Agents Chemother 1981; 20: 66
Amsterdam D. Instrumentation for antimicrobic susceptibility testing: yesterday, today, and tomorrow. Diagn Microbiol Infect Dis 1988; 9: 167–78
Greenwood D. Unrealistic nature of the ‘MIC’. J Antimicrob Chemother 1976; 2: 312
Thornsberry C, Gavan TL, Sherris JC, et al. Laboratory evaluation of a rapid, automated susceptibility testing system: report of a collaborative study. Antimicrob Agents Chemother 1975; 7: 466
Zinner SH, Blaser J, Gaya H. Laboratory support for choosing and monitoring antimicrobial therapy in severely ill patients. Am J Med 1986; 80 Suppl. 5C: 59–63
Nightingale J. Clinical limitations of in vitro testing of microorganism susceptibility. Am J Hosp Pharm 1987; 44: 131–7
Neu HC, Saha G, Chin N-X. Comparative in vitro activity and β-lactamase stability of FK482, a new oral cephalosporin. Antimicrob Agents Chemother 1989; 33: 1795–1800
Bryson HM, Brogden RN. Cefetamet pivoxil. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1993; 45: 589–621
Frampton JE, Brogden RN, Langtry HD, et al. Cefpodoxime proxetil. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential. Drugs 1992; 44: 889–917
Barry AL. Procedure for testing antibiotics in agar media: theoretical considerations. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore: Williams and Wilkins, 1980: 1–23
Gilbert ON, Skutschere E, Ireland P, et al. Effect of the concentrations of magnesium and calcium on the in vitro susceptibility of Pseudomonas aeruginosa to gentamicin. J Infect Dis 1971; 124 Suppl.: S37–S45
Relier LB, Schoenknecht FD, Kenny MA, et al. Antibiotic susceptibility testing of Pseudomonas aeruginosa: selection of a control strain and criteria for magnesium and calcium content in media. J Infect Dis 1974; 130: 454–63
Tomasz A. On the mechanism of the irreversible antimicrobial effects of β-lactams. Philos Trans R Soc Lond Biol 1980; 289: 303
Hamilton-Miller JMT, Ramsay J. Synergism between β-lactam antibiotics: a test of theoretical predictions made with Staphylococcus aureus. J Med Microbiol 1973; 6: 377
Yourassowsky E, Van der Linden MP, Crokaert F. Comparative kill and growth rates determined with cefdinir and cefaclor and with Streptococcus pneumoniae and β-lactamase-producing Haemophilus influenzae. Antimicrob Agents Chemother 1992; 36: 46–9
Klastersky J. Empiric treatment of infections in neutropenic patients with cancer. Rev Infect Dis 1983; 5 Suppl. 1: S21
NCCLS. National Committee for Clinical Laboratory Standards. Methods for determining bactericidal activity of antimicrobial agents: proposed guideline. NCCLS Publication No. M26-P. Villanova, PA, NCCLS, 1987
Goessens WHF, Fontijne P, Michel MF. Factors influencing detection of tolerance in Staphylococcus aureus. Antimicrob Agents Chemother 1982; 22: 364
Sabath LD, Laverdiere M, Wheeler N, et al. A new type of penicillin resistance in Staphylococcus aureus. Lancet 1977; 1: 443
MacLowry JD, Witebsky FG. Critical reflections on current problems associated with susceptibility testing and monitoring of antimicrobial therapy. Antimicrob Newsletter 1987; 4: 77–84
Goessens WHF. Basic mechanisms of bacterial tolerance of antimicrobial agents. Eur J Clin Microbiol Infect Dis 1993; 12 Suppl. 1: 9–12
Rozenberg-Arska M, Fabius GTJ, Beens-Dekkers MAAJ, et al. Antibiotic sensitivity and synergism of ‘penicillin-tolerant’ Staphylococcus aureus. Chemotherapy 1979; 25: 352
Jones RN, Erwin ME, Gooding BB. Interpretive criteria for disk diffusion tests using 5-microgram cefdinir disks with rapidly growing clinical isolates. J Clin Microbiol 1992; 30: 1022–3
Jones RN, Erwin ME. Haemophilus test medium interpretive criteria for disk diffusion susceptibility tests with cefdinir, cefetamet, cefmetazole, cefpodoxime, cefdaloxime (RU29246, HR-916 metabolite), and trospectomycin. Diagn Microbiol Infect Dis 1992; 15: 693–701
Barrett MS, Jones RN. Susceptibility testing interpretive criteria and drug stability for cefdinir, cefetamet, and cefpodoxime against Neisseria gonorrhoeae. Diagn Microbiol Infect Dis 1992; 15: 685–91
NCCLS. National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. 5th ed. Approved standard. NCCLS document M2-A5. Vol. 13, no. 24: Villanova, PA, NCCLS, 1993
McDonald PJ, Craig WA, Kunin CM. Persistent effect of antibiotics on Staphylococcus aureus after exposure for limited periods of time. J Infect Dis 1977; 135: 217–23
Bundtzen RW, Gerber AU, Cohn DL, et al. Postantibiotic suppression of bacterial growth. Rev Infect Dis 1981; 3: 217–23
Vogelman BS, Craig WA. Postantibiotic effects. J Antimicrob Chemother 1985; 15 Suppl. A: 37–46
Ebert SC. Characterization of the postantibiotic effect. Clin Pharm 1992; 11: 876–7
Blandino G, Caccamo F, Di Marco R, et al. Bactercidal activity and postantibiotic effect of cefdinir (CI 983, FK 482) against selected pathogens. Drugs Exp Clin Res 1992; 18: 319–27
Craig W. Relevance of animal models for clinical treatment. Eur J Clin Microbiol Infect Dis 1993; 12 Suppl. 1: 55–7
Rouse MS, Tallan BM, Henry NK, et al. Animal models as predictors of outcome of therapy with broad spectrum cephalosporins. J Antimicrob Chemother 1992; 29 Suppl. A: 39–45
Leitner F, Chisholm DR, Tsai YH, et al. BL-S640, a cephalosporin with a broad spectrum of antibacterial activity: bioavailability and therapeutic properties in rodents. Antimicrob Agents Chemother 1975; 7: 306
Miraglia GJ, Renz KJ, Gadebusch HH. Comparison of the chemotherapeutic and pharmacodynamic activities of cephradine, cephalothin, and cephaloridine in mice. Antimicrob Agents Chemother 1973; 3: 270
O’Callaghan CH, Kirby SM. Some cephalosporins in clinical use and their structure-activity relationships. Postgrad Med J 1970; 46 Suppl.: 9
Fare LR, Actor P, Sachs C, et al. Comparative serum levels and protective activity of parenterally administered cephalosporins in experimental animals. Antimicrob Agents Chemother 1974; 6: 150
Zak O, Tosch W, Sande MA. Correlation of antibacterial activities of antibiotics in vitro and in animal models of infection. J Antimicrob Chemother 1985; 15 Suppl. A: 273–82
Anderson ET, Young LS, Hewitt WL. Simultaneous antibiotic levels in ‘breakthrough’ Gram-negative rod bacteremia. Am J Med 1976; 61: 493
Noone P, Parsons TMC, Pattison JR, et al. Experience in monitoring gentamicin therapy during treatment of Gram-negative sepsis. B Med J 1974; 1: 477
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wiedemann, B. Laboratory Testing of Cephalosporins. Clin. Drug Invest. 9 (Suppl 3), 11–21 (1995). https://doi.org/10.2165/00044011-199500093-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199500093-00004