Summary
Abstract
Pemetrexed is a multi-targeted antifolate that inhibits DNA and RNA synthesis. It is approved in the US and Europe for use with cisplatin in the treatment of malignant pleural mesothelioma (MPM) in patients who are not candidates for surgery. It is also approved in Europe, and has received accelerated approval from the US FDA, for the second-line treatment of locally advanced or metastatic NSCLC.
Pemetrexed has shown clinical activity against MPM and NSCLC, and has favorable tolerability when patients are supplemented with folic acid and cyanocobalamin (vitamin B12). In patients with refractory or recurrent NSCLC, it has similar activity to docetaxel, with better tolerability and lower treatment costs. In the treatment of unresectable MPM, pemetrexed plus cisplatin has superior efficacy to single-agent cisplatin and is the only chemotherapeutic regimen to significantly prolong survival.
Pharmacologica Properties
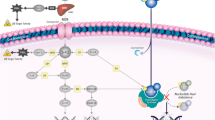
Pemetrexed is a pyrimidine-derived antifolate that primarily targets thymidylate synthase (TS), but also inhibits dihydrofolate reductase, and, to a lesser extent, glycinamide ribonucleotide formyl transferase (GARFT), and 5-aminoimidazole-4-carboxamide ribonucleotide formyl transferase (AICARFT). This limits the formation of both thymidylate and purines, thereby inhibiting DNA and RNA synthesis.
Pemetrexed enters the cell through both the reduced folate transporter and the higher affinity, but lower capacity, α-folate receptor. It is rapidly polyglutamated, increasing retention and enhancing the affinity of pemetrexed for, and inhibition of, TS, AICARFT, and GARFT. The multi-enzyme activity of pemetrexed may explain its cytotoxicity against cell lines that are resistant to other single-target antifolates and may underlie its activity against some chemotherapy-resistant cancers, such as MPM. Pemetrexed induces synchronization of the cell cycle in the S phase, which may generate synergistic cytotoxicity by sensitizing cells to radiotherapy and other chemotherapeutic agents.
Following a 10-minute infusion of pemetrexed 500 mg/m2, the mean peak plasma concentration was 78 μg/mL, the terminal elimination half-life was 3.2 hours, and the mean clearance was 63 mL/min/m2 in patients with advanced solid tumors. Pemetrexed is ≈81% bound to human plasma proteins, not metabolized significantly in humans, and primarily eliminated through renal excretion. The amount of drug eliminated in the first 24 hours following pemetrexed infusion is significantly higher in women than in men. The use of pemetrexed in patients with a creatinine clearance <45 mL/min is not recommended.
Therapeutic Efficacy
Pemetrexed demonstrated clinical activity in patients with MPM, and those who had previously received treatment for NSCLC in two well designed trials. In patients with MPM, the use of pemetrexed in combination with cisplatin significantly improved median survival time (primary endpoint) by 2.8 months and the 1-year survival rate by 12% compared with cisplatin monotherapy. Pemetrexed plus cisplatin recipients also experienced a longer median time to disease progression, a greater objective response rate, superior overall health-related quality of life, and greater symptomatic relief (pain, dyspnea, fatigue, anorexia, and cough) than recipients of cisplatin alone.
In patients with NSCLC who had received prior treatment, no significant differences in median survival time, 1-year survival rate, objective response rate, or symptom palliation were observed between pemetrexed and docetaxel monotherapy. Non-inferiority analysis indicated at least 52% of the survival benefit of docetaxel over best supportive care was maintained with pemetrexed.
Tolerability
Pemetrexed monotherapy, when supplemented with folic acid and cyanocobalamin, is generally well tolerated. In patients with NSCLC, the tolerability profile of pemetrexed is favorable in comparison with that of docetaxel,with a significantly lower incidence of neutropenia and febrile neutropenia and a similar frequency of nonhematologic adverse events. Pemetrexed recipients also had a lower rate of hospitalization for neutropenic fever than docetaxel recipients and a reduced requirement for supportive care that, in a UK analysis, resulted in a lower cost per patient.





Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
World Health Organization. Global cancer rates could increase by 50% to 15 million by 2020 [online]. Available from URL: http://www.who.int/mediacentre/releases/2003/pr27/en/print.html [Accessed 2004 Sep 29]
National Cancer Institute. Survelllance, Epidemiology, and End Results (SEER) Program Nov 2003 submission (1973–2001) [online]. Available from URL: http://www.seer.cancer.gov [Accessed 2004 Aug 20]
Carbone M, Kratzke RA, Testa JR. The pathogenesis of mesothelioma. Semin Oncol 2002 Feb; 29(1): 2–17
Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000 May; 18(10): 2095–103
Ruffie P, Feld R, Minkin S, et al. Diffuse malignant mesothelioma of the pleura in Ontario and Quebec: a retrospective study of 332 patients. J Clin Oncol 1989 Aug; 7: 1157–68
Brenner J, Sordillo PP, Magill GB, et al. Malignant mesothelioma of the pleura: review of 123 patients. Cancer 1982; 49(11): 2431–5
National Cancer Institute. Non-small cell lung cancer (PDQ): treatment [online]. Available from URL: gov/cancerinfo/pdq/treatment/non-small-cell-lung/healthprofessional [Accessed 2004 Aug 1]
Sterman DH, Kaiser LR, Albelda SM. Advances in the treatment of malignant pleural mesothelioma. Chest 1999 Aug; 116(2): 504–20
Harrison TS, Scott LJ. Pemetrexed. Am J Cancer 2003; 2(5): 359–70
US Food and Drug Administration. FDA approves first drug for rare type of cancer [online]. Available from URL: http://www.fda.gov/bbs/topics/NEWS/2004/NEW01018.html [Accessed 2004 Sep 2]
US Food and Drug Administration. New from CDER [online]. Available from URL: http://www.fda.gov/cder/ [Accessed 2004 Sep 2]
European Medicines Agency. Committee for Medicinal Products for Human Use European Public Assessment Report (EPAR). Alimta [online]. Available from URL: http://www. emea. eu. int/humandocs/Humans/EPAR/alimta/alimta.htm [Accessed 2004 Sep 28]
Shih C, Habeck LL, Mendelsohn LG, et al. Multiple folate enzyme inhibition: mechanism of a novel pyrrolopyrimidine-based antifolate LY231514 (MTA). Adv Enzyme Regul 1998; 38: 135–52
Shih C, Chen VJ, Gossett LS, et al. LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res 1997 Mar; 57(6): 1116–23
Taylor EC, Kuhnt D, Shih C, et al. A dideazatetrahydrofolate analogue lacking a chiral center at C-6,N-[4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyr- rolo[2,3-d]pyrimidin-5-yl)ethyi]benzoyl]-L-glutamic acid, is an inhibitor of thymidylate synthase. J Med Chem 1992; 35: 4450–4
Tonkinson JL, Marder P, Andis SL, et al. Cell cycle effects of antifolate antimetabolites: implications for cytotoxicity and cytostasis. Cancer Chemother Pharmacol 1997; 39(6): 521–31
Kindler HL. The pemetrexed/gemcitabine combination in pancreatic cancer. Cancer 2002 Aug 15; 95(4 Suppl.): 928–32
Calvert H, Bunn Jr PA. Future directions in the development of pemetrexed. Semin Oncol 2002 Apr; 29(2 Suppl. 5): 54–61
Zhao R, Babani S, Gao F, et al. The mechanism of transport of the multitargeted antifolate (MTA) and its cross-resistance pattern in cells with markedly impaired transport of methotrexate. Clin Cancer Res 2000 Sep; 6(9): 3687–95
Theti DS, Jackman AL. The role of a-folate receptor-mediated transport in the antitumor activity of antifolate drugs. Clin Cancer Res 2004 Feb; 10(3): 1080–9
Bueno R, Appasani K, Mercer H, et al. The a folate receptor is highly activated in malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2001 Feb; 121(2): 225–33
Habeck LL, Mendelsohn LG, Shih C, et al. Substrate specificity of mammalian folylpolyglutamate synthetase for 5,10-dideazatetrahydrofolate analogs. Mol Pharmacol 1995 Aug; 48(2): 326–33
Schultz RM, Chen VJ, Bewley JR, et al. Biological activity of the multitargeted antifolate, MTA (LY231514), in human cell lines with different resistance mechanisms to antifolate drugs. Semin Oncol 1999 Apr; 26(2 Suppl. 6): 68–73
Britten CD, Izbicka E, Hilsenbeck S, et al. Activity of the multitargeted antifolate LY231514 in the human tumor cloning assay. Cancer Chemother Pharmacol 1999; 44(2): 105–10
Giovannetti E, Mey V, Danesi R, et al. Synergistic cytotoxicity and pharmaco-genetics of gemcitabine and pemetrexed combination in pancreatic cancer cell lines. Clin Cancer Res 2004 May; 10(9): 2936–43
Tonkinson JL, Worzalla JF, Teng CH, et al. Cell cycle modulation by a multitarged antifolate, LY231514, increases the cytotoxicity and antitumor activity of gemcitabine in HT29 colon carcinoma. Cancer Res 1999 Aug; 59: 3671–6
Adjel AA, Erlichman C, Sloan JA, et al. Phase I and pharmacologic study of sequences of gemcitabine and the multitargeted antifolate agent in patients with advanced solid tumors. J Clin Oncol 2000 Apr; 18(8): 1748–57
Kano Y, Akutsu M, Tsunoda S, et al. Schedule-dependent synergism and antagonism between pemetrexed and paclitaxel in human carcinoma cell lines in vitro. Cancer Chemother Pharmacol 2004 Aug 31; 54(6): 505–13
Schultz RM, Dempsey JA. Sequence dependence of Alimta (LY231514, MTA) combined with doxorubicin in ZR-75-1 human breast carcinoma cells. Anti-cancer Res 2001 Sep-Oct; 21(5): 3209–14
Telcher BA, Chen V, Shih C, et al. Treatment regimens including the multitargeted antifolate LY231514 in human tumor xenografts. Clin Cancer Res 2000 Mar; 6(3): 1016–23
Backus HH, Pinedo HM, Wouters D, et al. Differences in the induction of DNA damage, cell cycle arrest, and cell death by 5-fluorouracil and antifolates. Oncol Res 2000; 12(5): 231–9
Liani E, Rothem L, Bunni MA, et al. Loss of folylpoly-gamma-glutamate synthetase activity is a dominant mechanism of resistance to polyglutamylation-dependent novel antifolates in multiple human leukemia sublines. Int J Cancer 2003 Feb 20; 103(5): 587–99
Wang Y, Zhao R, Goldman ID. Decreased expression of the reduced folate carrier and folypolyglutamate synthetase is the basis for acquired resistance to the pemetrexed antifolate (LY231514) in an L1210 murine leukemia cell line. Biochem Pharmacol 2003 Apr; 65(7): 1163–70
Wang Y, Zhao R, Goldman ID. Characterization of a folate transporter in HeLa cells with a low pH optimum and high affinity for pemetrexed distinct from the reduced folate carrier. Clin Cancer Res 2004 Sep 15; 10 (18 Pt 1): 6256–64
Zhao R, Hanscom M, Chattopadhyay S, et al. sciective preservation of pemetrexed pharmacological activity in HeLa cells lacking the reduced folate carrier: association with the presence of a secondary transport pathway. Cancer Res 2004 May 1; 64(9): 3313–9
Zhao R, Titus S, Gao F, et al. Molecular analysis of murine leukemia cell lines resistant to 5, 10-dideazatetrahydrofolate identifies several amino acids critical to the function of folylpolyglutamate synthetase. J Biol Chem 2000 Aug 25; 275(34): 26599–606
Mauritz R, Peters GJ, Priest DG, et al. Multiple mechanisms of resistance to methotrexate and novel antifolates in human CCRF-CEM leukemia cells and thelr implications for folate homeostasis. Biochem Pharmacol 2002 Jan; 63(2): 105–15
Rhee MS, Ryan TJ, Galivan J. Glutamyl hydrolase and the multitargeted antifolate LY231514. Cancer Chemother Pharmacol 1999; 44(5): 427–32
Welsh SJ, Titley J, Brunton L, et al. Comparison of thymidylate synthase (TS) proteln up-regulation after exposure to TS inhibitors in normal and tumor cell lines and tissues. Clin Cancer Res 2000; 6(6): 2538–46
Smith PG, Marshman E, Calvert AH, et al. Prevention of thymidine and hypoxanthine rescue from MTA (LY231514) growth inhibition by dipyridamole in human lung cancer cell lines. Semin Oncol 1999 Apr; 26(2 Suppl. 6): 63–7
Niyikiza C, Baker SD, Seitz DE, et al. Homocystelne and methylmalonic acid: markers to predict and avoid toxicity from pemetrexed therapy. Mol Cancer Ther 2002 May; 1(7): 545–52
Worzalla JF, Shih C, Schultz RM. Role of folic acid in modulating the toxicity and efficacy of the multitargeted antifolate, LY231514. Anticancer Res 1998; 18: 3235–40
Zhao R, Gao F, Goldman ID. Marked suppression of the activity of some, but not all, antifolate compounds by augmentation of folate cofactor pools within tumor cells. Biochem Pharmacol 2001 Apr 1; 61(7): 857–65
Rinaldi DA, Kuhn JG, Burris HA, et al. A phase I evaluation of multitargeted antifolate (MTA, LY231514), administered every 21 days, utilizing the modified continual reassessment method for dose escalation. Cancer Chemother Pharmacol 1999; 44(5): 372–80
Takimoto CH, Forero L, Baker SD, et al. Phase I and pharmacokinetic study of LY231514 (pemetrexed disodium, MTA) in renal dysfunction patients [abstract no. 41PD]. Ann Oncol 2002 Oct; 13Suppl. 5: 12
Sweeney C, Baker S, Murry D, et al. Pharmacokinetic (PK) and pharmacodynamic analysis of aspirin administered with multi-targeted antifolate (ALIMTA) [abstract no. 104]. Eur J Cancer 2001 Oct; 37Suppl. 6: 31
Hughes A, Calvert P, Azzabi A, et al. Phase I clinical and pharmacokinetic study of pemetrexed and carboplatin in patients with malignant pleural mesothelioma. J Clin Oncol 2002 Aug; 20(16): 3533–44
Thödtmann R, Depenbrock H, Dumez H, et al. Clinical and pharmacokinetic phase I study of multitargeted antifolate (LY231514) in combination with cisplatin. J Clin Oncol 1999 Oct; 17(10): 3009–16
Woodland JM, Barnett CJ, Dorman DE, et al. Metabolism and disposition of the antifolate LY231514 in mice and dogs. Drug Metab Dispos 1997 Jun; 25(6): 693–700
US Food and Drug Administration. Alimta® pemetrexed for injection [online]. Available from URL: http://www.fda.gov/cder/foi/label/2004/0216771bl.pdf [Accessed 2004 Sep 2]
Orlando M, Wozniak A, Janne P, et al. Pemetrexed alone or in combination with cisplatin in previously treated patients with malignant pleural mesothelioma (MPM): outcomes of an expanded access program (EAP) [abstract no. 7195]. Proc Am Soc Clin Oncol 2004; 23: 661
Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003 Jul; 21(14): 2636–44
Wozniak AJ, Janne P, Belani CP, et al. Pemetrexed in combination with cisplatin in the treatment of chemonaive patients with malignant pleural mesothelioma (MPM): outcomes on expanded access program (EAP) [abstract no. 7192]. Proc Am Soc Clin Oncol 2004; 23: 661
Hanna N, Shepherd FA, Fosscila FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004 May; 22(9): 1589–97
Smit EF, Mattson K, von Pawel J, et al. ALIMTA (pemetrexed disodium) as second-line treatment of non-small-cell lung cancer: a phase II study. Ann Oncol 2003; 14: 455–60
Symanowski JT, Rusthoven J, Nguyen B, et al. Multiple regression analysis of prognostic variables for survival from the phase III study of pemetrexed + cisplatin vs. cisplatin in malignant pleural mesotheliomia [abstract no. 2602]. Proc Am Soc Clin Oncol 2003; 22: 647
Gralla RJ, Hollen PJ, Liepa AM, et al. Improving quality of life in patients with malignant pleural mesothelioma: results of the randomized pemetrexed + cisplatin vs. cisplatin trial using the LCSS-meso instrument [abstract no. 2496]. Proc Am Soc Clin Oncol 2003 May; 22: 621. Plus oral presentation at the 39th Annual Meeting of the American Society of Clinical Oncology; 2003 31 May–3 Jun; Chicago
Gralla R, Hollen P, Liepa A, et al. Documenting symptom palliation with chemotherapy using the LCSS-Meso: results from the randomized trial of pemetrexed plus cisplatin vs cisplatin alone in patients with malignant pleural mesothelioma [abstract no. 905]. Eur J Cancer Suppl 2003 Sep; 1(5): 272
Bunn PA, Paoletti P, Niyikiza C, et al. Vitamin B12 and folate reduce toxicity of AlimtaTM (pemetrexed disodium, LY231514, MTA), a novel antifolate/antimetabolite [abstract no. 300]. Proc Am Soc Clin Oncol 2001; 20 (Pt. 1): 76a
Rusthoven JJ, Eisenhauer E, Butts C, et al. Multitargeted antifolate LY231514 as first-line chemotherapy for patients with advanced non-small-cell lung cancer: a phase II study. J Clin Oncol 1999 Apr; 17(4): 1194–9
Bushill-Matthews L, Jackson D, Aristides M, et al. Reducing health care burden for the treatment of toxicity associated with pemetrexed or docetaxel in patients with advanced non-small cell lung cancer who previously recelved chemotherapy: application to the UK setting [abstract no. 769]. Eur J Cancer Suppl 2003 Sep; 1(5): 231
Eli Lilly and Company. Alimta recelves positive endorsement for lung cancer approval from FDA advisory committee [online]. Available from URL: http://newsroom.lilly.com/news/Product/2004-7-27_alimta_odac_positively_endorsed.html [Accessed 2004 Aug 21]
European Medicines Agency. Alimta product information [online]. Available from URL: http://www.emea.eu.int/humandocs/PDFs/EPAR/alimta/H-564-PI-en.pdf [Accessed 2004 Nov 18]
Kleln G, Powers A, Croce C. Association of SV40 with human tumors. Oncogene 2002 Feb 14; 21(8): 1141–9
Butchart EG. Contemporary management of malignant pleural mesothelioma. Oncologist 1999; 4(6): 488–500
Rusch VW, Venkatraman E. The importance of surgical staging in the treatment of malignant pleural mesothelioma. J Thorac Cardiovasc Surg 1996 Apr; 111(4): 815–25
Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999; 117(1): 54–65
National Cancer Institute. Malignant mesothelioma (PDQ): treatment [online]. Available from URL: http://www.cancer.gov/cancertopics/pdq/treatment/malignantmesothelioma/healthprofessional [Accessed 2004 Aug 25]
Ball DL, Cruickshank DG. The treatment of malignant mesothelioma of the pleura: Review of a 5-year experience, with special reference to radiotherapy. Am J Clin Oncol 1990; 13(1): 4–9
Boutin C, Schlesser M, Frenay C, et al. Malignant pleural mesothelioma. Eur Respir J 1998 Oct; 12(4): 972–81
Ryan CW, Herndon J, Vogelzang NJ. A review of chemotherapy trials for malignant mesothelioma. Chest 1998; 113(1 Suppl.): 66S–73S
Tomek S, Manegold C. Chemotherapy for malignant pleural mesothelioma: past results and recent developments. Lung Cancer 2004; 45S: S103–19
Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol 2004 Jan; 22(2): 330–53
Shepherd FA. Second-line chemotherapy for non-small cell lung cancer. Expert Rev Anticancer Ther 2003 Aug; 3(4): 435–42
US National Institutes of Health. Alimta (pemetrexed) in patients with non-small cell lung cancer who have failed a prior platinum-containing chemotherapy [online]. Available from URL: http://clinicaltrials.gov/show/NCT00078260 [Accessed 2004 Oct 28]
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: H.A. Burris, The Sarah Cannon Research Institute, Nashville, Tennessee, USA; M. Del Tacca, Department of Oncology, Transplants and Advanced Technologies in Medicine, Division of Pharmacology and Chemotherapy, University of Pisa, Pisa, Italy; I.D. Goldman, Albert Einstein College of Medicine Cancer Center, New York, New York, USA; A.R. Hanauske, Department of Medicine, Division of Medical Oncology, General Hospital St Georg, Hamburg, Germany; M. Karthaus, Med. Klinik II, Ev. Johannes Krankenhaus, Bielefeld, Germany; D.A. Rinaldi, Louisiana Oncology Associates, Lafayette, Louisiana, USA; D. Von Hoff, Arizona Cancer Center, Tuscon, Arizona, USA; A. Wozniak, Hudson-Webber Cancer Research Center, Detroit, Michigan, USA.
Data Selection
Sources: Medical literature published in any language since 1980 on pemetrexed, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘pemetrexed’ or ‘LY-231514’ and (‘non small cell lung cancer’ or ‘mesothelioma’). EMBASE search terms were ‘pemetrexed’ or ‘LY-231514’ and (‘non small cell lung cancer’ or ‘mesothelioma’). AdisBase search terms were ‘pemetrexed’ or ‘LY231514’ and (‘non-small-cell-lung-cancer’ or ‘*mesothelioma’ ). Searches were last updated 18 Nov 2004.
Selection: Studies in patients with mesothelioma or non-small-cell lung cancer who received pemetrexed. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Pemetrexed, mesothelioma, non-small-cell lung cancer, pharmacodynamics, pharmacokinetics, therapeutic use.
Rights and permissions
About this article
Cite this article
Robinson, D.M., Keating, G.M. & Wagstaff, A.J. Pemetrexed. Am J Cancer 3, 387–399 (2004). https://doi.org/10.2165/00024669-200403060-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024669-200403060-00006