Abstract
Background: Long-acting methylphenidate formulations provide control of attention-deficit hyperactivity disorder (ADHD) symptoms for up to 12 hours; however, not all formulations have rapid onset of therapeutic effect, which is essential for providing symptom control during morning hours. The primary objective of this randomized, double-blind, crossover study was to assess the efficacy of dexmethylphenidate extended release (ER) versus placebo by measuring the change from pre-dose to 0.5 hours post-dose on the Swanson, Kotkin, Agler, M-Flynn and Pelham (SKAMP) rating scale.
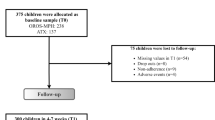
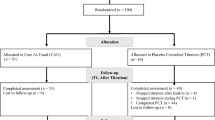
Methods: Eighty-six children (6–12 years) with ADHD diagnosed using the DSM-IV criteria were randomized to receive dexmethylphenidate ER 20 mg/day or placebo, sequentially, for 7 days, with the final dose administered in a laboratory classroom setting on day 7 of each treatment period. The primary efficacy comparison was change in the SKAMP-Combined score from pre-dose to 0.5 hours post-dose, with additional secondary assessments at 1, 2, 4, 6 and 8 hours post-dose. Secondary efficacy measures included change from pre-dose at all timepoints in the SKAMP-Attention and SKAMP-Deportment, Math Test-Attempted and Math Test-Correct scores, and change from baseline on the Conners’ ADHD/DSM-IV Scale for Parents (CADS-P). In an exploratory analysis, a daily diary card was completed by parents on the children’s in-home behaviour before school. Safety was assessed by occurrence of adverse events, monitoring of vital signs and interpretation of ECGs.
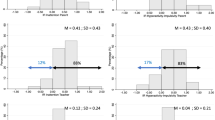
Results: Significant improvements were noted at 0.5 hours and at all timepoints post-dose throughout the 8-hour laboratory classroom day for dexmethylphenidate ER vs placebo in the primary outcome measure of the SKAMP-Combined scores (p < 0.001), as well as SKAMP-Attention, SKAMP-Deportment, Math Test-Attempted and Math Test-Correct scores (p < 0.05). The changes from baseline in CADS-P scores were significantly greater with dexmethylphenidate ER than placebo (−16.382 vs −4.622; p < 0.001). Responses to all diary questions indicated significant improvement with dexmethylphenidate ER treatment versus placebo (all p < 0.001). The most common adverse events were abdominal pain (dexmethylphenidate ER 3.5%; placebo 4.7%), headache (dexmethylphenidate ER 3.5%; placebo 2.3%) and increased appetite (dexmethylphenidate ER 0%; placebo 3.5%).
Conclusion: Compared with placebo, once-daily dexmethylphenidate ER 20 mg provided rapid and significant improvement at 0.5 hours post-dose in attention, deportment and academic performance, which was sustained for 8 hours post-dose. Overall, once-daily dexmethylphenidate ER 20 mg was well tolerated. In an analysis of parental assessment of diary responses, children appeared more organized, and morning preparation for school was smoother and less frustrating with once-daily dexmethylphenidate ER compared with placebo.








Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Goldman LS, Genel M, Bezman RJ, et al. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. JAMA 1998; 279: 1100–7
Biederman J, Faraone SC. Attention-deficit hyperactivity disorder. Lancet 2005; 367: 237–48
Sawyer MG, Whaites L, Rey JM, et al. Health-related quality of life of children and adolescents with mental disorders. J Am Acad Child Adolesc Psychiatry 2002; 41: 530–7
DeVeaugh-Geiss J, Conners CK, Sarkis EH, et al. GW320659 for the treatment of attention-deficit/hyperactivity disorder in children. J Am Acad Child Adolesc Psychiatry 2002; 41: 914–20
Bastiaansen D, Koot HM, Ferdinand RF, et al. Quality of life in children with psychiatric disorders: self, parent, and clinician report. J Am Acad Child Adolesc Psychiatry 2004; 43: 221–30
Klassen A, Miller A, Fine S. Health-related quality of life in children and adolescents who have a diagnosis of attention-deficit/hyperactivity disorder. Pediatrics 2004; 114: E541–7
Quinn D, Wigal S, Swanson J, et al. Comparative pharmacodynamics and plasma concentrations of d-threo-methylphenidate hydrochloride after single doses of d-threo-methylphenidate hydrochloride and d,l-threo-methylphenidate hydrochloride in a double-blind, placebo-controlled, crossover laboratory school study in children with attention-deficit/ hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2004; 43: 1422–9
Wigal S, Swanson JM, Feifel D, et al. A double-blind, placebo-controlled trial of dexmethylphenidate hydrochloride and d,l-threo-methylphenidate hydrochloride in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2004; 43: 1406–14
Pelham WE, Gnagy EM, Burrows-Maclean L, et al. Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics 2001; 107: E105
Swanson JM, Wigal SB, Wigal T, et al. A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (COMACS Study). Pediatrics 2004; 113: E206–16
Lopez F, Silva R, Pestreich L, et al. Comparative efficacy of two once-daily methylphenidate formulations (Ritalin LA and Concerta) and placebo in children with attention deficit hyperactivity disorder across the school day. Pediatr Drugs 2003; 5: 545–55
Silva R, Muniz R, Pestreich LK, et al. Efficacy of two long-acting methylphenidate formulations in children with attention-deficit/hyperactivity disorder in a laboratory classroom setting. J Child Adolesc Psychopharmacol 2005; 15: 637–54
Swanson J. Compliance with stimulants for attention-deficit/ hyperactivity disorder: issues and approaches for improvement. CNS Drugs 2003; 17: 117–31
Wilens TE, Gignac M, Swezey A, et al. Characteristics of adolescents and young adults with ADHD who divert or misuse their prescribed medications. J Am Acad Child Adolesc Psychiatry 2006; 45: 408–14
Lage M, Hwang P. Effect of methylphenidate formulation for attention deficit hyperactivity disorder on patterns and outcomes of treatment. J Child Adolesc Psychopharmacol 2004; 4(4): 575–81
Thiruchelvam D, Charach A, Schachar RJ. Moderators and mediators of long-term adherence to stimulant treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry 2001; 40(8): 922–8
Living with ADHD: a survey among US parents of children/ teenagers and adult sufferers. Conducted by Harris Interactive for Feinstein Kean Healthcare, 2007 Mar 27 [online]. Available from URL: http://www.harrisinteractive.com/harris_poll/ [Accessed 2008 Mar 31]
Srinivas NR, Hubbard JW, Quinn D, et al. Enantioselective pharmacokinetics and pharmacodynamics of dl-threo-methylphenidate in children with attention deficit hyperactivity disorder. Clin Pharmacol Ther 1992; 52: 561–8
Markowitz JS, DeVane CL, Pestreich LK, et al. Comprehensive in vitro screening of d-, l- and dl-threo-methylphenidate: an exploratory study. J Child Adolesc Psychopharmacol 2006; 16: 687–98
Novartis Pharmaceuticals. Focalin® XR: prescribing information [online]. Available from URL: http://www.pharma.us.novartis.com/product/pi/pdf/focalinXR.pdf [Accessed 2008 Mar 20]
Silva RR, Munix R, Pestreich L, et al. Dexmethylphenidate extended release capsules in children with attention-deficit/ hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2008; 47: 199–208
Silva RR, Muniz R, McCague K, et al. Treatment of children with attention-deficit/hyperactivity disorder (ADHD): results of a randomized, multicenter, double-blind, crossover study of extended-release dexmethylphenidate and d,l-methylphenidate and placebo in a laboratory classroom setting. Psychopharmacol Bulletin 2008; 41: 19–33
Muniz R, Brams M, Mao A, et al. Efficacy and safety of extended-release dexmethylphenidate compared with d,l-methylphenidate and placebo in the treatment of children with attention deficit hyperactivity disorder: a 12-hour laboratory classroom study. J Child Adolesc Psychopharmacol 2008; 18: 248–56
Swanson JM, Kinsbourne M, Roberts W, et al. A time-response analysis of effect of stimulant medication on the learning ability of children referred for hyperactivity. Pediatrics 1978; 61: 21–9
Swanson JM, Wigal S, Greenhill L, et al. Analog classroom assessment of Adderall in children with ADHD. J Am Acad Child Adolesc Psychiatry 1998; 37: 519–26
Wigal S, Gupta S, Guinta D, et al. Reliability of the SKAMP Rating Scale in a laboratory school setting. Psychopharmacol Bull 1998; 34: 47–53
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association, 1994: 78–85
Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36: 980–8
Swanson JM, Wigal S, Greenhill L, et al. Subjective and objective measures of the pharmacodynamic effects of Adderall in the treatment of children with ADHD in a controlled laboratory classroom setting. Psychopharmacol Bull 1998; 34: 55–61
Swanson JM, Wigal SB, Udrea D, et al. Evaluation of individual subjects in the analog classroom setting: I. Examples of graphical and statistical procedures for within-subject ranking of responses to different delivery patterns of methylphenidate. Psychopharmacol Bull 1998; 34: 825–32
Conners CK, Parker JD, Sitarenios G, et al. The revised Conners’ Parent Rating Scale: factor, structure, reliability, and criterion validity. J Abnormal Child Psychology 1998; 26: 257–68
Kelsey DK, Sumner CR, Casat CD, et al. Once-daily atomoxetine treatment for children with attention deficit/hyperacitvity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics 2004; 114: E1–8
Acknowledgements
This study was funded by Novartis Pharmaceuticals Corporation and reports the following involvement: design and conduct of the study; collection, management, analysis and interpretation of data; and preparation, review and approval of the manuscript.
Matthew Brams, MD, has been a speaker, consultant and advisory board member for Novartis and Shire, and has received grant-research support from Novartis, Shire and Eli Lilly.
Rafael Muniz, MD, is an employee of Novartis Pharmaceuticals Corporation and owns stock options as part of his employment.
Ann Childress, MD, has been a speaker, consultant and advisory board member for Novartis and Shire; speaker for Bristol-Myers Squibb; and received grant-research support from Novartis, Shire, Abbott, Astra-Zeneca, Somerset, Eli Lilly, Johnson & Johnson, Neuropharm and Bristol-Meyers Squibb.
John Giblin, MD, FAAP, has been a speaker and consultant for Novartis and Shire; and has received grant-research support from Novartis, Shire, Abbott, Esai, GlaxoSmithKline, Johnson & Johnson, Ortho-McNeill, Sanofi-Aventis, Cephalon, New River Pharmaceuticals, Wyeth and Pfizer.
Alice Mao, MD, has been a speaker for Novartis, Eli Lilly, Bristol-Meyers Squibb, Astra-Zeneca, Sepracor and Shire; consultant for Eli Lilly, Novartis, and Shire; and receives grant-research support from Novartis.
John Turnbow, MD, has been a speaker and advisory board member for Novartis, UCB Pharma and Shire; receives grant-research support from Novartis, Shire and Sanofi-Aventis; and has had past grant-research support from Celltech (now UCB Pharma).
Mary Borrello, RN, is an employee of Novartis and owns stock options as part of her employment.
Kevin McCague, MA, is an employee of Novartis and owns stock options as part of his employment.
Frank Lopez, MD, has been a speaker, consultant and advisory board member for Novartis, Shire and Eli Lilly; and receives research grants from Noven, Novartis, Shire, New River Pharmaceuticals, Celltech-Medeva, Bristol-Myers Squibb, Wyeth, GlaxoSmithKline and Cephalon.
Raul Silva, MD, reports no conflicts of interest since 15 December 2006; prior to this date he was a speaker for Novartis, AstraZeneca and Janssen and received grant-research support from Novartis, Forest and Celgene.
The authors gratefully acknowledge Bonnie Brien of Novartis Pharmaceuticals Corporation who assisted in the preparation of a first draft of this article based on an author-approved outline, and also assisted in implementing author revisions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brams, M., Muniz, R., Childress, A. et al. A Randomized, Double-Blind, Crossover Study of Once-Daily Dexmethylphenidate in Children with Attention-Deficit Hyperactivity Disorder. CNS Drugs 22, 693–704 (2008). https://doi.org/10.2165/00023210-200822080-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200822080-00006