Abstract
Background and Objectives
Concerns exist regarding the rising use of methylphenidate. A double-blind, placebo-controlled methylphenidate titration (PCT) for children with attention-deficit/hyperactivity disorder (ADHD) has shown potential to improve titration (i.e., detection of placebo responders and larger ADHD symptom improvement) in experimental settings. This study aims to determine if these advantages can be transferred to clinical settings.
Method
Children (aged 5–13 years) with an ADHD diagnosis and an indication to start methylphenidate (MPH) treatment were recruited. Participants were randomized to PCT or care as usual (CAU) in a 1:1 ratio followed by a 7-week randomized controlled trial (T1) and 6-month, naturalistic, open-label follow-up (T2). Parents, teachers, and physicians rated ADHD symptoms, ADHD medication use, MPH dosing, and treatment satisfaction using questionnaires.
Results
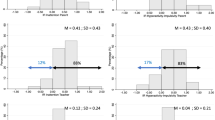
A total of 100 children were enrolled and randomized to PCT (n = 49) or CAU (n = 51). In the PCT group, we found 8.2% placebo responders, 16.3% non-responders, and 65.3% responders to MPH. With PCT compared with CAU, a significantly larger number of children discontinued MPH (T1: 24.5 vs 5.9%, p = 0.009; T2: 41.7 vs 10.4%, p < 0.001) and refrained from using other pharmacological treatment (T1: 20.4 vs 3.9%, p = 0.013; T2: 20.83 vs 6.25%, p = 0.002). At both timepoints, there were no significant differences between the groups in the average dose of MPH, ADHD symptoms, or treatment satisfaction.
Conclusions
PCT can be used to improve detection of children who do not benefit from MPH, and may therefore potentially reduce overtreatment of ADHD with MPH.



Similar content being viewed by others
References
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–8. https://doi.org/10.1176/ajp.2007.164.6.942.
Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. Attention-deficit/hyperactivity disorder. Nat Rev. 2015;1:15020. https://doi.org/10.1038/nrdp.2015.20.
Shaw M, Hodgkins P, Caci H, Young S, Kahle J, Woods AG, Arnold LE. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med. 2012;2012(10):99. https://doi.org/10.1186/1741-7015-10-99.
Luo Y, Halperin JM, Li X, Weibman D. A review of heterogeneity in attention deficit/hyperactivity disorder (ADHD). Front Hum Neurosci. 2019. https://doi.org/10.3389/fnhum.2019.00042.
National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management NICE guideline. 2018. http://www.nice.org.uk/guidance/ng87. Accessed 20 Nov 2022.
Wolraich ML, Hagan JF Jr, Allan C, Chan E, Davison D, Earls M, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2019;144(4): e20192528.
Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727–38. https://doi.org/10.1016/S2215-0366(18)30269-4.
Greenhill LL, Abikoff HB, Arnold LE, Cantwell DP, Conners CK, et al. Medication treatment strategies in the MTA study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry. 1996;35(10):1304–13. https://doi.org/10.1097/00004583-199610000-00017.
Vitiello B, Severe JB, Greenhill LL, et al. Methylphenidate dosage for children with ADHD over time under controlled conditions: lessons from the MTA. J Am Acad Child Adolesc Psychiatry. 2001;40(2):188–96. https://doi.org/10.1097/00004583-200102000-00013.
Vertessen K, Luman M, Swanson JM, Bottelier M, et al. Methylphenidate dose-response in children with ADHD: evidence from a double-blind, randomized placebo-controlled titration trial. Eur Child Adolesc Psychiatry. 2023. https://doi.org/10.1007/s00787-023-02176-x.
Greenhill LL, Swanson JM, Vitiello B, et al. Impairment and deportment responses to different methylphenidate doses in children with ADHD: the MTA titration trial. J Am Acad Child Adolesc Psychiatry. 2001;40(2):180–7. https://doi.org/10.1097/00004583-200102000-00012.
Medori R, Ramos-Quiroga JA, Casas M, Kooij JJ, Niemelä A, et al. A randomized, placebo-controlled trial of three fixed dosages of prolonged-release OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63(10):981–9. https://doi.org/10.1016/j.biopsych.2007.11.008.
Farhat LC, et al. The effects of stimulant dose and dosing strategy on treatment outcomes in attention-deficit/hyperactivity disorder in children and adolescents: a meta-analysis. Mol Psychiatry. 2022;27(3):1562–72. https://doi.org/10.1038/s41380-021-01391-9.
Rommelse N, Bunte T, Matthys W, Anderson E, Buitelaar J, Wakschlag L. Contextual variability of ADHD symptoms: embracement not erasement of a key moderating factor. Eur Child Adolesc Psychiatry. 2015;24(1):1–4. https://doi.org/10.1007/s00787-014-0665-1.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013.
Canadian ADHD Resource Alliance (CADDRA). Canadian ADHD practice guidelines, 4th edn. Toronto: CADDRA; 2018. https://www.caddra.ca/wp-content/uploads/CADDRA-Guidelines-4th-Edition_-Feb2018.pdf. Accessed 20 Nov 2022.
Coghill D, Seth S. Effective management of attention-deficit/hyperactivity disorder (ADHD) through structured re-assessment: the Dundee ADHD Clinical Care Pathway. Child Adolesc Psychiatry Ment Health. 2015. https://doi.org/10.1186/s13034-015-0083-2.
The MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/ hyperactivity disorder. Arch Gen Psyciatry. 1999;56(12):1073–86.
Kovshoff H, Williams S, Vrijens M, et al. The decisions regarding ADHD management (DRAMa) study: uncertainties and complexities in assessment, diagnosis and treatment, from the clinician’s point of view. Eur Child Adolesc Psychiatry. 2012;21(2):87–99. https://doi.org/10.1007/s00787-011-0235-8.
Hodgkins P, Shaw M, Coghill D, Hechtman L. Amfetamine and methylphenidate medications for attention-deficit/ hyperactivity disorder: complementary treatment options. Eur Child Adolesc Psychiatry. 2012;21:477–92. https://doi.org/10.1007/s00787-012-0286-5.
Kamimura-Nishimura KI, Brinkman WB, Froehlich TE. Strategies for improving ADHD medication adherence. Curr Psychiatry. 2019;18(8):25.
Luman M, Goos V, Oosterlaan J. Instrumental learning in ADHD in a context of reward: intact learning curves and performance improvement with methylphenidate. J Abnorm Child Psychol. 2015;43(4):681–91. https://doi.org/10.1007/s10802-014-9934-1.
Froehlich TE, Epstein JN, Nick TG, Melguizo Castro MS, et al. Pharmacogenetic predictors of methylphenidate dose-response in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(11):1129–39. https://doi.org/10.1016/j.jaac.2011.08.002.
Scheres A, Oosterlaan J, Swanson J, et al. The effect of methylphenidate on three forms of response inhibition in boys with AD/HD. J Abnorm Child Psychol. 2003;31(1):105–20. https://doi.org/10.1023/a:1021729501230.
Geladé K, Bink M, Janssen TWP, et al. An RCT into the effects of neurofeedback on neurocognitive functioning compared to stimulant medication and physical activity in children with ADHD. Eur Child Adolesc Psychiatry. 2017;26(4):457–68. https://doi.org/10.1007/s00787-016-0902-x.
Reichart CG, Wals M, Hillegers M. Vertaling K-sads. Utrecht: HC Rümke Groep; 2000.
Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–8. https://doi.org/10.1097/00004583-199707000-00021.
Oosterlaan J, Baeyens D, Scheres A, Antrop I, Roeyers H, Sergeant JA. Vragenlijst voor gedragsproblemen bij kinderen 6–16 jaar, Handleiding. Amsterdam: Harcourt Publisher; 2008.
Praktikon. https://www.bergop.info.
Swanson JM, Schuck S, Porter M, et al. Categorical and dimensional definitions and evaluations of symptoms of ADHD: history of the SNAP and the SWAN rating scales. Int J Educ Psychol Assess. 2012;10(1):51–70.
Young DJ, Levy F, Martin NC, Hay DA. Attention deficit hyperactivity disorder: a Rasch analysis of the SWAN rating scale. Child Psychiatry Hum Dev. 2009;40(4):543–59. https://doi.org/10.1007/s10578-009-0143-z.
Twisk JWR. Analysis of data from a randomized controlled trial. A practical guide. Heidelberg: Springer; 2021.
Twisk JWR. Applied mixed model analysis. Cambridge: Cambridge University Press; 2019.
Gray JR, Kagan J. The challenge of predicting which children with attention deficit-hyperactivity disorder will respond positively to methylphenidate. J Appl Dev Psychol. 2000;21(5):471–89.
Johnston BA, Coghill D, Matthews K, Steele JD. Predicting methylphenidate response in attention deficit hyperactivity disorder: a preliminary study. J Psychopharmacol. 2015;29(1):4–30. https://doi.org/10.1177/0269881114548438.
Cortese S, Newcorn JH, Coghill D. A practical, evidence-informed approach to managing stimulant-refractory attention deficit hyperactivity disorder (ADHD). CNS Drugs. 2021. https://doi.org/10.1007/s40263-021-00848-3.
Faraone SV, Newcorn JH, Cipriani A, et al. Placebo and nocebo responses in randomised, controlled trials of medications for ADHD: a systematic review and meta-analysis. Mol Psychiatry. 2022;27(1):212–9. https://doi.org/10.1038/s41380-021-01134-w.
Acknowledgements
We would like to thank all participating children, parents, teachers, and physicians for their participation in the study. We thank all research interns for their valuable support in data collection. We are grateful to all participating centers for their help in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study is part of the project ‘Reduce and optimize methylphenidate use in children and adolescents with ADHD’ (Grant p3119), funded by Innovation Fund of Health Insurances (Innovatiefonds Zorgverzekeraars). The funder/sponsor did not participate in the work.
Conflict of interest
Karen Vertessen has been involved in a clinical trial sponsored by Takeda. The other authors have no conflicts of interest relevant to this article to disclose.
Ethics approval
The local ethics committee approved the study (METC Amsterdam UMC, 2016.594) and the study was registered prospectively in the Dutch trial register (NL8121).
Consent to participate
Parents and children older than 11 years provided signed informed consent.
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The code used to analyze the data in the current study are available from the corresponding author on reasonable request.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by MB, RS, KV and AW. Professor JT was the statistical expert for this study. The first draft of the manuscript was written by KV and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript and agree to be accountable for the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vertessen, K., Luman, M., Bet, P. et al. Improving Methylphenidate Titration in Children with Attention-Deficit/Hyperactivity Disorder (ADHD): A Randomized Controlled Trial Using Placebo-Controlled Titration Implemented in Clinical Practice. Pediatr Drugs (2024). https://doi.org/10.1007/s40272-023-00604-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s40272-023-00604-8