Abstract
Successful treatment strategies for patients with traumatic brain injury (TBI) remain elusive despite standardised clinical treatment guidelines, improved understanding of mechanisms of cellular response to trauma, and a decade of clinical trials aimed at identifying therapeutic agents targeted at mediators of secondary injury.
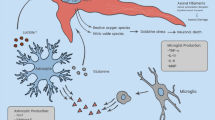
The information explosion relative to mechanisms of secondary injury has identified several potential targets for intervention. Depending on the type of injury to the brain and the intensity and the success of resuscitation, necrosis, apoptosis, inflammatory and excitotoxic cellular damage can be seen. These same processes may continue postinjury, depending on the adequacy of clinical care. Each of these mechanisms of cellular damage can initiate a cascade of events mediated by endogenous signals that lead to secondary neurological injury.
Several factors contributed to the failure of earlier clinical trials. Now that these have been recognised, a positive impact on future drug development in TBI has been realised. Both the US and Europe have organised brain injury consortiums where experts in the treatment of TBI provide insight into study design, implementation, conduct and oversight in conjunction with the pharmaceutical industry. Consequently, future clinical trials of new investigational treatments have greater potential for identifying therapies of merit in specific populations of patients with TBI.
Pharmacological strategies under investigation are targeting sites involved in the secondary cascade that contribute to overall poor outcome following the primary injury. These treatments include ion channel antagonists including calcium channel antagonists, growth factors, antioxidants, stem cells, apoptosis inhibitors, and inhibitors of other signal modulators.
In conclusion, the complexity of TBI pathology and the mechanisms contributing to secondary injury present unique therapeutic challenges. Appropriate research targets for intervention continue to be investigated, however, the likelihood of improving outcomes with a single approach is extremely small. There is a need for collaborative efforts to investigate the optimal time for drug administration and the logical sequence or combination of treatments that will ultimately lead to improved neurological outcomes in this population.


Similar content being viewed by others
References
NIH Consensus Development Panel on Rehabilitation of Persons with Traumatic Brain Injury. Rehabilitation of persons with traumatic brain injury. JAMA 1999; 282(10): 974–83
Murray GD, Teasdale GM, Braakman R, et al. The European Brain Injury Consortium survey of head injuries. ActaNeuro-chir (Wien) 1999; 141: 223–36
Bullock R. Experimental drug therapies for head injury. In: Narayan RK, Wilberger JE, Povlishock JT, editors. Neuro-trauma. New York: McGraw-Hill, 1996: 375–91
Bullock MR, Lyeth BG, Muizelaar JP. Current status of neuroprotection trials for traumatic brain injury: lessons from animal models and clinical studies. Neurosurgery 1999; 45(2): 207–20
Teasdale GM, Graham DI. Craniocerebral trauma: protection and retrieval of the neuronal population after injury. Neurosurgery 1998; 45(4): 723–38
Gopinath SP, Robertson CS, Contant CF, et al. Jugular venous desaturation and outcome after head injury. J Neurol Neurosurg Psychiatry 1994; 57(6): 717–23
Chestnut RM, Marshall SB, Piek J, et al. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir Suppl (Wien) 1993; 59: 121–5
Young B, Ott L, Rapp R, et al. The effect of nutritional support on outcome of severe head injury. J Neurosurg 1987; 67: 668–76
Bredesen DE. Apoptosis: overview and signal transduction pathways. J Neurotrauma 2000; 17(10): 801–10
The Brain Trauma Foundation. Management and prognosis of severe traumatic brain injury: part I guidelines for the management of severe traumatic brain injury. J Neurotrauma 2000; 17: 449–553
Roberts I, Schierhout G, Alderson P. Absence of evidence for the effectiveness of five interventions routinely used in the intensive care management of severe head injury: a systematic review. J Neurol Neurosurg Psychiatry 1998; 65: 729–33
Mass AIR, Dearden M, Teasdale GM, et al. EBIC-guidelines for management of severe head injury in adults: European Brain Injury Consortium. Acta Neurochir (Wien) 1997; 139: 286–94
Alderson P, Roberts I. Corticosteroids in acute traumatic brain injury: systematic review of randomised controlled trials. BMJ 1997; 314: 1855–9
Schierhout G, Roberts I. Prophylactic antiepileptic agents after head injury: a systematic review. J Neurol Neurosurg Psychiatry 1998; 64: 108–12
Robertson CS, Valadka AB, Hannay J, et al. Prevention of secondary ischemic insults after severe head injury. Crit Care Med 1999; 27(10): 2086–95
Young B, Runge JW, Waxman KS, et al. Effects of pegorgotein on neurologic outcome of patients with severe head injury. JAMA 1996; 276(7): 538–43
Morris GF, Bullock R, Marshall SB, et al. Failure of the competitive N-methyl-D-aspartate antagonist selfotel (CGS 19755) in the treatment of severe head injury: results of two phase III clinical trials. The Selfotel Investigators. J Neurosurg 1999; 91: 737–43
Mass AIR, Steyerberg E, Murray GD, et al. Why have recent trials of neuroprotective agents in head injury failed to show convincing efficacy? A pragmatic analysis and theoretical considerations. Neurosurgery 1999; 44(6): 1286–98
Marshall LF, Maas A, Marshall SB, et al. A multicenter trial on the efficacy of using tirilazad mesylate in cases of head injury. J Neurosurg 1998; 89: 519–25
Marshall LF, Marshall SB. Pitfalls and advances from the international tirilazad trial in moderate and severe head injury. J Neurotrauma 1995; 12(5): 929–32
The Brain Trauma Foundation. Management and prognosis of severe traumatic brain injury. Part II: early indicators of prognosis in severe traumatic brain injury. J Neurotrauma 2000; 17(6/7): 557–627
Marmarou A. Conduct of head injury trials in the United States: the American Brain Injury Consortium (ABIC). Acta Neurochir Suppl (Wien) 1996; 66: 118–21
Reinert MM, Bullock R. Clinical trials in head injury. Neurol Res 1999; 21(4): 330–8
Doppenberg EMR, Choi CS, Bullock R. Clinical trials in traumatic brain injury: what can we learn from previous studies? Ann N Y Acad Sci 1999; 890: 305–22
Lees KR. Cerestat and other NMDA antagonists in ischemic stroke. Neurology 1997; 49Suppl. 4: S66–9
Winquist RJ, Kerr S. Cerebral ischemia-reperfusion injury and adhesion. Neurology 1997; 49Suppl. 4: S23–6
Clark WM. Cytokines and reperfusion injury. Neurology 1997; 49Suppl. 4: S10–4
Zipfel GJ, Lee J-M, Choi DW Reducing calcium overload in the ischemic brain. N Engl J Med 1999; 341(20): 1543–4
Olney JW. Neurotoxicity of excitatory amino acids. In: McGeer EG, editor. Kainic acid as a tool in neurobiology. New York: Raven Press, 1978: 95–121
McIntosh TK, Vink R, Soares H, et al. Effects of the N-methyl-D-aspartate receptor blocker MK-801 on neurologic function after experimental brain injury. J Neurotrauma 1989; 6(4): 247–59
Saikumar P, Dong Z, Mikhailov V, et al. Apoptosis: definition, mechanisms, and relevance to disease. Am J Med 1999; 107: 489–503
Eldadah BA, Faden AI. Caspase pathways, neuronal apoptosis, and CNS injury. J Neurotrauma 2000; 17(10): 811–30
Graham SH, Chen J, Clark RSB. Bcl-2 family gene products in cerebral ischemia and traumatic brain injury. J Neurotrauma 2000; 17(10): 831–42
Leslie JB, Watkins WD. Eicosanoids in the central nervous system. J Neurosurg 1985; 63: 659–68
McClain C, Cohen D, Phillips R, et al. Increased plasma and ventricular fluid interleukin 6 levels in patients with head injury. J Lab Clin Med 1991; 118: 225–31
Kossmann T, Hans VH, Imhof HG, et al. Intrathecal and serum interleukin 6 and the acute phase response in patients with severe traumatic brain injuries. Shock 1995; 4: 311–7
Bell MJ, Kochanek PM, Doughty LA, et al. Interleukin 6 and interleukin 10 in cerebrospinal fluid after severe traumatic brain injury in children. J Neurotrauma 1997; 14: 451–7
Fan L, Young PR, Barone FC, et al. Experimental brain injury induces expression of interleukin-1beta mRNA in the rat brain. Brain Res Mol Brain Res 1995; 30: 125–30
Shohami E, Novikov M, Bass R, et al. Closed head injury triggers early production of TNF alpha and IL6 by brain tissue. J Cereb Blood Flow Metab 1994; 14: 615–9
Wang X, Yue T, Barone F. Concomitant cortical expression of TNF-alpha and IL-1beta mRNAs follows early response gene expression in transient focal ischemia. Mol Chem Neuropathol 1994; 23: 103–14
Shohami E, Bass R, Wallach D, et al. Inhibition of tumor necrosis factor alpha (TNFalpha) activity in rat brain is associated with cerebroprotection after closed head injury. J Cereb Blood Flow Metab 1996; 16(3): 378–84
Feuerstein G, Wang X, Barone FC. Cytokines in brain ischemia - the role of TNF alpha. Cell Mol Neurobiol 1998; 18(6): 695–701
Knoblach SM, Fan L, Faden AI. Early neuronal expression of tumor necrosis factor-alpha after experimental brain injury contributes to neurological impairment. J Neuroimmunol 1999; 95(1-2): 115–25
Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: role of tumor necrosis factor-alpha. Cerebrovasc Brain Metab Rev 1994; 6(4): 341–60
Shohami E, Novikov M, Bass R, et al. Closed head injury triggers early production on TNF-alpha and IL-6 by brain tissue. J Cereb Blood Flow Metab 1994; 14(4): 615–9
McKeating EG, Andrews P, Signorini DF, et al. Transcranial cytokine gradients in patients requiring intensive care after acute brain injury. Br J Anaesth 1997; 78(5): 520–3
Sei Y, Vitkovic L, Yokoyama MM. Cytokines in the central nervous system: regulatory roles in neuronal function, cell death and repair. Neuroimmunomodulation 1995; 2: 121–33
Toulmond S, Vige X, Fage D, et al. Local infusion of interleukin 6 attenuates the neurotoxic effects of NMDA on rat striatal cholinergic neurons. Neurosci Lett 1992; 144: 49–52
Campbell IL, Abraham CR, Masliah E, et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A 1993; 90: 10061–5
Rothwell NJ, Relton JK. Involvement of cytokines in acute neurodegeneration in the CNS. Neurosci Biobehav Rev 1992; 17: 217–27
Selmaj KW, Farroq M, Norton WT, et al. Proliferation of astrocytes in vitro in response to cytokines. J Immunol 1990; 144: 129–35
Wang X, Feuerstein GZ. Induced expression of adhesion molecules following focal ischemia. J Neurotrauma 1995; 12: 825–32
Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic-ischemic brain damage. Ann Neurol 1986; 19: 105–11
Teasdale GM, Barman P. Neuroprotection in head injury. In: Reilly P, Bullock R, editors. Head Injury. London: Chapman & Hall, 1997: 423–38
Foster AC. Channel blocking drugs for the NMDA receptor. In: Meldrum BS, editor. Excitate amino acid antagonists. Oxford: Blackwell Scientific 1991: 164-79
Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma 1996; 13: 25–34
Luer MS, Rhoney D, Hughes M, et al. New pharmacologic strategies for acute neuronal injury. Pharmacotherapy 1996; 16(5): 830–48
Bullock R. Opportunities for neuroprotective drugs in clinical management of head injury. J Emerg Med 1993; 11: 23–30
Dixon CE, Clifton GL, Lighthall JW, et al. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods 1991; 39: 253–62
Nicholls D, Atwell D. The release and uptake of excitate amino acids. Trends Pharmacol Sci 1990; 11: 462–8
Carter C, Benavidies J, Dana C, et al. Noncompetitive NMDA receptor antagonists acting on the polyamine site. In: Meldrum BS, editor. Excitate acid amino antagonists. Oxford: Black-well Scientific, 1991: 136–63
Bullock R. Strategies for neuroprotection with glutamate antagonists. Ann N Y Acad Sci 1999; 890: 272–8
Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J Neurotrauma 2000; 17(10): 843–56
Zipfel GJ, Babcock DJ, Lee -M, et al. Neuronal apoptosis after CNS injury: the roles of glutamate and calcium. J Neurotrauma 2000; 17(10): 857–70
Roberts GW, Gentleman SM, Lynch A, et al. Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 1994; 57: 419–25
Hovda DA, Lee SM, Smith ML, et al. The neurochemical and metabolic cascade following brain injury: moving from animal models to man. J Neurotrauma 1995; 12: 903–6
Sullivan PG, Thompson MD, Scheff SW. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp Neurol 1999; 160: 226–34
Reiss P, Bareyre FM, Cheney J, et al. Effect of cyclosporin A on behavioral outcome after severe brain injury in rats [abstract]. J Neurotrauma 1999; 10: 1016
Alessandri B, Di X, DeFord M, et al. Cyclosporin A improves learning after lateral fluid percussion injury in rats [abstract]. J Neurotrauma 1999; 10: 981
Scheff SW, Sullivan PG. Cyclosporin A significantly amerliorates cortical damage following experimental traumatic brain injury in rodents. J Neurotrauma 1999; 16: 783–92
Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma 2000; 17(10): 871–90
Beckman JS, Campbell GA, Hannan CJ, et al. Involvement of Superoxide and xanthine oxidase with death due to cerebral ischemia-induced seizures in gerbils. In: Rotilio G, editor. Superoxide and Superoxide dismutase in chemistry, biology, and medicine. New York: Elsevier, 1986: 602–7
Chieueh C. Neuroprotective properties of nitric oxide. Ann N Y Acad Sci 1999; 890: 301–11
Hall ED, Kupina N, Althaus JS. Peroxynitrite scavengers for the acute treatment of traumatic brain injury. Ann N Y Acad Sci 1999; 890: 462–8
Yakovlev AG, Faden AI. Molecular strategies in CNS injury. J Neurotrauma 1995; 12: 767–77
Mattson MP, Scheff SW. Endogenous neuroprotection factors and traumatic brain injury: mechanisms of action and implications for therapy. J Neurotrauma 1994; 11: 3–33
Hicks RR, Li C, Zhang L, et al. Alterations in BDNF and trkB mRNA levels in the cerebral cortex following experimental brain trauma in rats. J Neurotrauma 1999; 16(6): 501–10
Hicks RR, Numan S, Dhillon HS, et al. Alterations in BDNF and NT-3 mRNAs in rat hippocampus after experimental brain trauma. Brain Res Mol Brain Res 1997 48(2): 401–6
Sandberg Nordqvist AC, von Holst H, Holmin S, et al. Increase of insulin-like growth factor (IGF)-1, IGF binding protein-2 and -4 mRNAs following cerebral contusion. Brain Res Mol Brain Res 1996; 38(2): 285–93
Dore S, Bastianetto S, Kar S, et al. Protective and rescuing abilities of IGF-1 and some putative free radical scavengers against beta-amyloid-inducing toxicity in neurons. Ann N Y Acad Sci 1999; 890: 356–64
Hatton J, Rapp R, Kudsk KA, et al. Intravenous insulin-like growth factor-I (IGF-1) in moderate-to-severe head injury: a phase II safety and efficacy trial. J Neurosurg 1997; 86: 779–86
Tasker RC. Pharmacological advance in the treatment of acute brain injury. Arch Dis Child 1999; 81(1): 90–5
Sacchetti ML, Toni D, Fiorelli M, et al. The concept of combination therapy in acute ischemic stroke. Neurology 1997; 49 Suppl. 4: 570–4
Robertson GS, Crocker S, Nicholson DW, et al. Neuroprotection by the inhibition of apoptosis. Brain Pathol 2000; 10(2): 283–92
Cosi C, Marien M. Implication of poly(ADP-ribose) polymerase (PARP) in neurodegeneration and brain energy metabolism. Ann N Y Acad Sci 1999; 890: 227–40
Jolkkonen J, Puurunen K, Koistinaho J, et al. Neuroprotection by the alpha2-adrenoceptor agonist, dexmedetomidine, in rat focal cerebral ischemia. Eur J Pharmacol 1999; 372: 31–6
Cherian L, Chacko G, Goodman JC, et al. Cerebral hemodynamic effects of phenylephrine and L-arginine after cortical impact injury. Crit Care Med 1999; 27(11): 2512–7
Chen Y, Shohami E, Constantini S, et al. Rivastigmine, a brain selective ACEsterase inhibitor ameliorates cognitive and motor deficits induced by closed-head injury in the mouse. J Neurotrauma 1998; 15(4): 231–7
Pike BR, Hamm R. Post-injury administration of BIBN99, a selective muscarinic M2 receptor antagonist, improves cognitive performance following traumatic brain injury in rats. Brain Res 1995; 686(1): 37–42
Pruneau D, Chorny I, Benkovitz V, et al. Effect of LF 16-0687MS, a new nonpeptide bradykinin B2 receptor antagonist, in a rat model of closed head trauma. J Neurotrauma 1999; 16(11): 1057–65
Stover JF, Dohse NK, Unterberg AW. Significant reduction in brain swelling by administration of nonpeptide kinin B2 receptor antagonist LF 16-0687MS after controlled cortical impact injury in rats. J Neurosurg 2000; 92(5): 853–9
Narotam PK, Rodell TC, Nadvis S, et al. Traumatic brain contusions: a clinical role for the kinin antagonist CP-0127. Acta Neurochir (Wien) 1998; 140(8): 793–802
Marmarou A, Nichols J, Burgess J, et al. ABIC study group. Effects of the bradykinin antagonist Bradycor (deltibant, CP-0127) in severe traumatic brain injury: results of a multi-center, randomised, placebo-controlled trial. J Neurotrauma 1999; 16(6): 431–44
Strauss KI, Marshall R, Narayan RK. COX2 increases acutely and chronically in neurons and astrocytes following traumatic brain injury in the rat [abstract]. J Neurotrauma 1999; 1005
Dash PK, Mach S, Moore AN. Regional expression and role of cyclooxygenase-2 following experimental traumatic brain injury. J Neurotrauma 2000; 17(1): 69–81
Koyfman L, Kaplanski J, Artru AA, et al. Inhibition of cyclooxygenase 2 by numesulide decreases prostaglandin E2 formation but does not alter brain edema or clinical recovery after closed head injury in rats. J Neurosurg Anesthesiol 2000; 12(1): 44–50
Moises JP, Marcheselli VL, Bazan NG. Brain injury induces COX-2 gene expression: inhibition by a PAF antagonist [abstract]. J Neurotrauma 1999; 1005
Bentzer P, Mattiasson GJ, MxcIntosh T, et al. Effect of prostacyclin on cortical lesion volume following experimental brain injury in the rat [abstract]. J Neurotrauma 1999; 979
Baskaya MK, Dogan A, Rao AM, et al. Neuroprotective effects of citicoline on brain edema and blood-brain barrier breakdown after traumatic brain injury. J Neurosurg 2000; 92(3): 448–52
Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phenycyclidine and related drugs. Science 1989; 244: 1360–2
Fix AS, Horn JW, Wightman KA, et al. Neuronal vacuolization and necrosis induced by the noncompetitive NMDA antagonists MK801 (dizocilpine maleate): a light and electron microscopic evaluation of the rat retrosplenial cortex. Exp Neurol 1993; 123(2): 204–15
Hogg S, Perron C, Barneoud P, et al. Neuroprotective effect of eliprodil: attenuation of a conditioned freezing deficit induced by traumatic injury of the right parietal cortex in the rat. J Neurotrauma 1998; 15(7): 545–53
Okiyama K, Smith D, White WF, et al. Effects of the novel NMDA antagonists CP-98,113, CP-101,581 and CP-101,606 on cognitive function and regional cerebral edema following experimental brain injury in the rat. J Neurotrauma 1997; 14(4): 211–22
Bullock MR, Merchant R, Carmack CA, et al. An open-label study of CP-101,606 in subjects with a severe traumatic head injury of spontaneous intracerebral hemorrhage. Ann N Y Acad Sci 1999; 890: 51–8
Merchant RE, Bullock MR, Carmack CA, et al. A double-blind, placebo-controlled study of the safety, tolerability and pharmacokinetics of CP-101,606 in patients with a mild or moderate traumatic brain injury. Ann N Y Acad Sci 1999; 890: 42–50
Muir KW, Grosset D, Lees KR. Clinical pharmacology of CNS 1102 in volunteers. Ann N Y Acad Sci 1999; 890: 279–89
Wagstaff A, Teasdale G, Clifton G, et al. The cerebral hemodynamic and metabolic effects of the noncompetitive NMDA antagonist CNS 1102 in humans with severe head injury. Ann N Y Acad Sci 1999; 890: 332–3
Cawley MJ, Marburger RK, Earl GL. Investigational neuroprotective drugs in traumatic brain injury. J Neurosci Nurs 1998; 30(6): 369–74
The North American Glycine Antagonist in Neuroprotection (GAIN) Investigators. Phase II studies of the glycine antagonist GV150526 in acute stroke: the North American experience. Stroke 2000; 31: 358–65
Di X, Bullock R. Effect of the novel high-affinity glycine-site N-methyl-D-aspartate antagonist ACEA-1021 on 1125MK-801 binding after subdural hematoma in the rat: an in vivo autoradiographic study. J Neurosurg 1996; 85(4): 655–61
Tsuchida E, Bullock R. The effect of the glycine site-specific N-methyl-D-aspartate antagonist ACEA1021 on ischemic brain damage caused by acute subdural hematoma in the rat. J Neurotrauma 1995; 12(3): 279–88
Palmer GC, Cregan EF, Borrelli AR, et al. Neuroprotective properties of the uncompetitive NMDA receptor antagonist remacemide hydrochloride. Ann N Y Acad Sci 1995; 765: 236–47
Black MA, Tremblay R, Mealing GA, et al. The desglycinyl metabolite of remacemide hydrochloride is neuroprotective in cultured rat cortical neurons. J Neurochem 1996; 66(3): 989–95
Kawaguchi K, Graham S. Neuroprotective effects of the glutamate release inhibitor 629C89 in temporary middle cerebral artery occlusion. Brain Res 1997; 749(1): 131–4
Okiyama K, Smith D, Gennarelli TA, et al. The sodium channel blocker and glutamate release inhibitor BW1003C87 and magnesium attenuate regional cerebral edema following experimental brain injury in the rat. J Neurochem 1995; 64(2): 802–9
Vornov JJ, Wozniak K, Lu M, et al. Blockade of NAALADase: a novel neuroprotective strategy based on limiting glutamate and elevating NAAG. Ann N Y Acad Sci 1999; 890: 400–5
Borowicz KK, Luszczki J, Szadkowski M, et al. Influence of LY 300164, an antagonist of AMPA/kainate receptors, on the anticonvulsant activity of clonazepam. Eur J Pharmacol 1999; 380: 67–72
Heath DL, Vink R. Traumatic brain axonal injury produces sustained decline in intracellular free magnesium concentration. Brain Res 1996; 738(1): 150–3
Bareyre FM, Saatman K, Helfaer MA, et al. Alterations in ionized and total blood magnesium after experimental traumatic brain injury: relationship to neurobehavioral outcome and neuroprotective efficacy of magnesium chloride. J Neurochem 1999; 73(1): 271–80
Heath DL, Vink R. Blood-free magnesium concentration declines following graded experimental traumatic brain injury. Scand J Clin Lab Invest 1998; 58(2): 161–6
Heath DL, Vink R. Brain free magnesium concentration is predictive of motor outcome following traumatic axonal brian injury in rats. Magnes Res 1999; 12(4): 269–77
McIntosh TK, Vink R, Yamakami I, et al. Magnesium protects against neurological deficit after brain injury. Brain Res 1989; 482(2): 252–60
Heath DL, Vink R. Magnesium sulphate improves neurologic outcome following severe closed head injury in rats. Neurosci Lett 1997; 228(3): 175–8
Heath DL, Vink R. Optimization of magnesium therapy after severe diffuse axonal brain injury in rats. J Pharmacol Exp Ther 1999; 288(3): 1311–6
Heath DL, Vink R. Neuroprotective effects of MgSO4 and MgC12 in closed head injury: a comparative phosphorus NMR study. J Neurotrauma 1998; 15(3): 183–9
Alexiou T, Crane L, Vink R. Increased mortality in female rats after severe diffuse traumatic brain injury is significantly attenuated by magnesium administration. Neurosci Res Commun 2000; 26(1): 1–8
Heath DL, Vink R. Improved motor outcome in response to magnesium therapy received up to 24 hours after traumatic diffuse axonal brain injury in rats. J Neurosurg 1999; 90(3): 504–9
Muir KW, Lees K. Dose optimization of intravenous magnesium sulfate after acute stroke. Stroke 1998; 29: 918–23
Shohami E, Gallily R, Mechoulam R, et al. Cytokine production in the brain following closed head injury: dexanabinol (HU-211) is a novel TNF-alpha inhibitor and an effective neuroprotectant. J Neuroimmunol 1997; 72(2): 169–77
Biegon A, Joseph A. Development of HU-211 as a neuroprotectant for ischemic brain damage. Neurol Res 1995; 17(4): 275–80
Shohami E, Novikov M, Bass R. Long-term effect of HU-211, a novel non-competitive NMDA antagonist, on motor and memory functions after closed head injury in the rat. Brain Res 1995; 674(1): 55–62
Brewster ME, Pop E, Foltz RL, et al. Clinical pharmacokinetics of escalating intravenous doses of dexabinol (HU-211), a neuroprotectant agent, in normal volunteers. Int J Clin Pharmacol Ther 1997; 35(9): 361–5
Pop E. Dexanabinol Pharmos. Curr Opin Investig Drugs 2000 Dec; 1(4): 494–503
Garcia-Estrada J, Luquin S, Fernandes AM, et al. Dehydroepi-androsterone, pregnenolone and sex steroids down-regulate reactive astroglia in the male rat brain after a penetrating brain injury. Int J Dev Neurosci 1999; 17(2): 145–51
Garcia-Segura LM, Wozniak A, Azcoitia I, et al. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience 1999; 89(2): 567–78
Zaulyanov LL, Green PS, Simpkins JW. Glutamate receptor requirement for neuronal death from anoxia-reoxygenation: an in vitro model for assessment of the neuroprotective effects of estrogens. Cell Mol Neurobiol 1999; 19(6): 705–18
Green PA, Gridley KE, Simpkins JW. Nuclear estrogen receptor-independent neuroprotection by estratrienes: a novel interaction with glutathione. Neuroscience 1998; 84(1): 7–10
Emerson CS, Headrick J, Vink R. Estrogen improves biochemical and neurologic oucome following traumatic brain injury in male rats, but not in females. Brain Res 1993; 608(l): 95–100
The European Study Group on Nimodipine in Severe Head Injury. A multicenter trial of the efficacy of nimodipine on outcome after severe head injury. J Neurosurg 1994; 80(5): 797–804
Teasdale G, Bailey I, Bell A, et al. A randomised trial of nimodipine in severe head injury: HIT I: British/Finnish Cooperative Head Injury Trial Group. J Neurotrauma 1992; 9Suppl. 2: S545–50
Murray GD, Teasdale G, Schmitz H. Nimodipine in traumatic subarachnoid haemorrhage: a re-analysis of the HIT I and HIT II trials. Acta Neurochir (Wien) 1996; 138(10): 1163–7
Compton JS, Lee T, Jones NR, et al. A double-blinded placebo controlled trial of the calcium entry blocking drug, nicardipine, in the treatment of vasospasm following severe head injury. Br J Neurosurg 1990; 4: 9–16
Samii A, Badie H, Fu K, et al. Effects of an N-type calcium channel antagonist (SNX 111; ziconotide) on calcium-45 accumulation following fluid-percussion injury. J Neurotrauma 1999; 16(10): 879–92
McBurney RN, Daly D, Fischer JB, et al. New CNS-specific calcium antagonists. J Neurotrauma 1992; 2 Suppl.: S531–43
Cheney JA, Brown A, Bareyre FM, et al. The novel compound LOE 908 attenuates acute neuromotor dysfunction but not cog-nitive impairment of cortical tissue loss following traumatic brain injury in rats. J Neurotrauma 2000; 17(1): 83–91
Uenishi H, Huang CS, Song JH, et al. Ion channel modulation as the basis for neuroprotective action of MS-153. Ann N Y Acad Sci 1999; 890: 385–99
Baldwin SA, Fugaccia I, Brown DR, et al. Blood-brain barrier breach following cortical contusion in the rat. J Neurosurg 1996; 85: 476–81
Liu J, Fanner Jr JD, Lane WS, et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 1991; 66: 807–15
O’Keefe SJ, Tamura J, Kincaid RL, et al. FK506 and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature 1992; 357: 692–4
Folbergrova J, Li PA, Uchino H, et al. Changes in the bioenergetic state of rat hippocampus during 2.5 minutes of ischemia and prevention of cell damage by cyclosporin A in hyperglycemic subjects. Exp Brain Res 1997; 114: 44–50
Li PA, Uchino H, Elmer E, et al. Amelioration by cyclosporin A of brain damage following 5 to 10 minutes of ischemia in rats subjected to preischemic hyperglycemia. Brain Res 1997; 753: 133–40
Shiga Y, Onodera H, Matsuo Y, et al. Cyclosporin A protects against ischemia-reperfusion injury in the brain. Brain Res 1992; 595: 145–8
Gogarten W, Van Aken H, Moskopp D, et al. A case of severe erebral trauma in a patient under chronic treatment with cyclosporin A. J Neurosurg Anesthesiol 1998; 10: 101–5
Chen YL, LeVraux V, Leneveu A, et al. Acute-phase response, interleukin-6 and alteration of cyclosporine pharmacokinetics. Clin Pharmacol Ther 1994; 55(6): 649–60
Kawamata T, Katayama Y, Maeda T, et al. Antioxidant, OPC-14117, attenuates edema formation and behavioral deficits following cortical contusion in rats. Acta Neurochir Suppl (Wein) 1997; 70: 191–3
Beit-Yannai E, Zhang R, Trembovler V, et al. Cerebroprotective effect of stable nitroxide radicals in closed head injury in the rat. Brain Res 1996; 717(1-2): 22–8
Kuroda S, Tsuchidate R, Smith ML, et al. Neuroprotective effects of a novel nitrone, NXY-059, after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab 1999; 19(7): 778–87
Kroppenstedt SN, Stroop R, Kern M, et al. Lubeluzole following traumatic brain injury in the rat. J Neurotrauma 1999; 16(7): 629–37
Beaumont A, Marmarou A. The effect of human corticotrophin releasing factor on the formation of post-traumatic cerebral edema. Acta Neurochir Suppl (Wien)1998; 71: 149–52
Deitrich WD, Alonso O, Busto R, et al. Posttreatment with intravenous basic fibroblast growth factor reduces histopathological damage following fluid-percussion brain injury in rats. J Neurotrauma 1996; 13(6): 309–16
Saatman KE, Contreras P, Smith DH, et al. Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp Neurol 1997; 147(2): 418–27
Young B, Hatton J, Rapp R, et al. Systemic and metabolic effects of insulin-like growth factor 1 and growth hormone in acute traumatic brain injury [abstract]. J Neurotrauma 1998; 15(1): 48
Park KI, Liu S, Flax JD, et al. Transplantation of neural progenitor and stem cells: developmental insights may suggest new therapies for spinal cord and other CNS dysfunction. J Neurotrauma 1999; 16(8): 675–87
Tzeng SF, Wu J. Responses of microglia and neural progenitors to mechanical brain injury. Neuroreport 1999; 10(11): 2287-92
Acknowledgements
Funding from the NIH has been awarded to investigate the effects of cyclosporin in TBI, effective as of June 2001. I would like to acknowledge the support of my family, particularly my father, in the preparation and effort of this article.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
There are several translational models of traumatic brain injury.[3] The animal model (rat, mouse,pig, cat, primate) and the mechanism for injury induction will determine which type of brain injury is similar in humans. These models and the associated pathological profile seen in humans are as follows:
-
Subdural haematoma — subdural haematoma, focal ischaemia, necrosis, delayed high intracranial pressure (ICP)
-
Intracerebral haematoma — intracerebral haematoma, focal ischaemia, high ICP
-
Middle cerebral artery occlusion — focal ischaemia
-
Global forebrain ischaemia — global ischaemia
-
Fluid percussion — contusion, diffuse axonal injury, cognitive effects, shearing trauma
-
Bodyweight-drop model — diffuse axonal injury, ischaemic necrosis
-
Controlled impact — contusion
Rights and permissions
About this article
Cite this article
Hatton, J. Pharmacological Treatment of Traumatic Brain Injury. Mol Diag Ther 15, 553–581 (2001). https://doi.org/10.2165/00023210-200115070-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200115070-00005