Abstract
Background
Streptococcus pneumoniae and Neisseria meningitidis group B are among the main causes of invasive bacterial meningitis infections in infants. Worldwide, these diseases lead to significant mortality, morbidity and costs. The societal impact is especially severe since the majority of cases occur in very young infants. A combination vaccine consisting of 9-valent conjugated pneumococcal and meningococcal B components is currently being developed. The aim of this study was to estimate the potential impact and cost effectiveness from the societal perspective of vaccinating infants in The Netherlands with this combination pneumococcal and meningococcal B vaccine versus no vaccination.
Methods
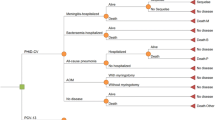
A Markov cycle model was developed using epidemiological and healthcare resource use data from 1996 to 2001. This model was used to project the annual costs, benefits and health gains associated with vaccinating all newborns. The base year for the costing was 2003 and all costs and health effects were discounted at 4%. The results of the analysis are expressed in costs per QALY and both probabilistic and univariate sensitivity analyses were used to identify the robustness of the results.
Results
Annually, an average of 755 cases of invasive pneumococcal and meningococcal B infection occurred in infants aged 0–10 years in The Netherlands. Introduction of the combination vaccine would prevent 201 cases of meningococcal B meningitis and 165 cases of invasive pneumococcal disease per year. Additionally, 3410 cases of pneumococcal pneumonia and 46 350 cases of otitis media would be prevented. Vaccination would save 35 lives per year and prevent 71 cases of severe sequelae. This translates into 860 life-years gained, or 1128 QALYs gained.
Alongside these health gains, vaccination would prevent €17 681 370 of direct medical and indirect costs attributable to meningococcal and pneumococcal infections in The Netherlands. Depending on vaccine price, cost effectiveness varied from €3160 (vaccine price per dose €20) to €32 170 (vaccine price €60 per dose) per QALY. Base-case cost effectiveness (vaccine price €40) was €17 700 per QALY. The model was most sensitive to changes in incidence, vaccine price and duration of protective efficacy.
Conclusion
Our results suggest that the introduction of a combination meningococcal B and pneumococcal vaccine into the Dutch infant vaccination programme is potentially cost effective compared with no vaccination.







Similar content being viewed by others
Notes
The expert panel consisted of members of the infectious diseases section of the Dutch Paediatric Society, which consists of representatives of most of the paediatric departments of university hospitals in The Netherlands. The expert panel was consulted on both the resource use of meningococcal and pneumococcal infections as well as assumptions on the severity of disease. Opinions of all specialists were noted and mean values were obtained for resource use (apart from number of inpatient days, which were derived from Prismant Healthcare).[24] For further details regarding the procedure, see Bos et al.[25]
References
Schildkamp RL, Lodder MC, Bijlmer HA. Clinical manifestation and course of meningococcal disease in 562 patients. Scand J Infect Dis 1996; 28: 47–21
Schuchat A, Robinson K, Wenger JD. Bacterial meningitis in the United States in 1995: active surveillance team. N Engl J Med 1997; 337: 970–977
Baraff LJ, Lee SI, Schriger DL. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr Infect Dis J 1993; 12 (5), 389–394
Kornelisse RF, Westerbeek CM, Spoor AB, et al. Pneumococcal meningitis in children: prognostic indicators and outcome. Clin Infect Dis 1995; 21 (6): 1390–1397
Miller E, Waight P, Efstratiou A, et al. Epidemiology of invasive and other pneumococcal disease in children in England and Wales 1996–1998. Acta Paediatr Suppl 2000; 89 (435): 11–16
Kaythy H, Eskola J. New vaccines for the prevention of pneumococcal infections. Emerg Infect Dis 1996; 2 (4): 289–298
Klein JO. The burden of otitis media. Vaccine 2000; 19 Suppl. 1: S2–S8
Zielhuis GA, Straatman H, Rach GH, et al. Analysis and presentation of data on the natural course of otitis media with effusion in children. Int J Epidemiol 1990; 19 (4): 1037–1044
Jodar L, Feavers IM, Salisbury D, et al. Development of vaccines against meningococcal diseases. Lancet 2005; 359: 1499–1508
Netherlands Reference Laboratory for Bacterial Meningitis (AMC/RIVM). Bacterial meningitis in The Netherlands: annual reports 1996–2001. Amsterdam, The Netherlands: RBM/Academic Medical Center Amsterdam, 2001
de Kleijn E, de Groot R, Lafeber A. Prevention of meningococcal serogroup B infections in children: a protein-based vaccine induces immunologic memory. J Infect Dis 2001; 184 (1): 98–102
Cartwright K, Morris K, Rumke HC. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (PorA) outer membrane proteins. Vaccine 2005; 17 (20–21): 2612–2619
Longworth E, Borrow R, Goldblatt D. Avidity maturation following vaccination with a meningococcal recorminant hexavalent PorA OMV vaccine in UK infants. Vaccine 2005; 20 (19-20): 2592–2596
Dutch Health Council. Pneumococcal and meningococcal vaccination [in Dutch]. The Hague, The Netherlands: Dutch Health Council, 2002
Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation cafIllaign against meningococcal serogroup C disease in the UK: a success story. Vaccine 2005; 20 Suppl. 1: S58–S67
Black S, Shinefield H. Safety and efficacy of the seven-valent pneumococcal conjugate vaccine: evidence from Northern California. Eur J Pediatr 2002; 161 Suppl. 2: S127–S131
Rennels MB, Edwards KM, Keyserling HL, et al. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRMI97 in United States infants. Pediatrics 1998; 101 (4 Pt 1): 604–611
Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001; 344 (6): 403–409
Dagan R, Givon-Lavi N, Zamir O, et al. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J Infect Dis 2005; 185 (7): 927–936
The World Bank. Immunisation at a glance [online]. Available from URL: http://wb1n0018.worldbank.org/HDNet/hddocs.nsf/%200/86eca35fe033893285256a42005e0dc6 [Accessed 2005 Jan 19]
Dodd D. Benefits of cormination vaccines: effective vaccination on a simplified schedule. Am J Manag Care 2005; 9 Suppl. 1: S6–S12
Lieu T A, Black S, Ray GT. The hidden costs of infant vaccination. Vaccine 2005; 19 (1): 33–41
Central Bureau for Statistics. Statistical yearbook 2002. The Hague, The Netherlands: SDU Publishers, 2002
Prismant Health Care. National medical registration of Dutch hospitals [in Dutch]. Utrecht, The Netherlands: SIG Healthcare, 1996
Bos JM, Rumke HC, Welte R, et al. Health economics of a hexavalent meningococcal outer-membrane vesicle vaccine in children: potential impact of introduction in the Dutch vaccination program. Vaccine 2001; 20 (1–2): 202–207
Vlug AE, van der LJ, Mosseveld BM, et al. Postmarketing surveillance based on electronic patient records: the IPCI project. Methods Inf Med 1999; 38 (4–5): 339–344
McCracken GH. Etiology and treatment of pneumonia. Pediatr Infect Dis J 2000; 19 (4): 373–377
Lebel MH, Kellner JD, Ford-Jones EL, et al. A pharmacoeconomic evaluation of 7-valent pneumococcal conjugate vaccine in Canada. Clin Infect Dis 2003; 36 (3): 259–268
Appelman CL, van Balen FA, van de Lisdonk EH. Dutch College of General Practitioners standard of care for otitis media. Utrecht, The Netherlands: Dutch College of General Practitioners, 1999
Stouthard MEA, Esselink-Bot ML, van Bonsel G. Weighing factors for diseases in The Netherlands. Amsterdam, The Netherlands: Institute for Social Health Care, Amsterdam Medical Center, 1997
Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000; 19 (3): 187–195
Centers for Disease Control and Prevention. Updated recommendations on the use of pneumococal conjugate vaccine: suspension of recommendation for third and fourth dose. MMWR Morb Mortal Wkly Rep 2005; 53: 177–178
Black S, Shinefield H, Ling S, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J 2005; 21 (9): 810–815
Bos JM, Rumke H, Welte R, et al. Epidemiologic impact and cost-effectiveness of universal infant vaccination with a 7-valent conjugated pneumococcal vaccine in the Netherlands. Clin Ther 2003; 25 (10): 2614–2630
Vermont CL, van Dijken HH, Kuipers AJ, et al. Cross reactivity of antibodies against PorA after vaccination with a meningococcal B outer membrane vesicle vaccine. Infect Immun 2005; 71: 1650–1655
National Health Tariffs Authority. Costing tariffs for medical specialists [in Dutch]. Utrecht, The Netherlands: National Health Tariffs Authority, 2001
Oostenbrink JB, Koopmanschap MA, Rutten FF. Guidelines for costing research: methods and reference prices for economic evaluation in healthcare [in Dutch]. Amstelveen, The Netherlands: College of Health Care Insurance, 2000
College of Health Care Insurance. Pharmaco-therapeutic compass 2001 [in Dutch]. Utrecht, The Netherlands: Roto Srneets Utrecht, 2001
College of Health Care Insurance. Diagnostic compass 2001–2002 [in Dutch]. Utrecht, The Netherlands: Roto Srneets Utrecht, 2001
Black S, Lieu TA, Ray GT, et al. Assessing costs and cost effectiveness of pneumococcal disease and vaccination within Kaiser Permanente. Vaccine 2000; 19 Suppl. 1: S83–S86
Stouthard MEA, Essink-Bot ML, Bonsel GJ. Methodology: disability weights for diseases. A modified protocol and results for a Western European region. Eur J Public Health 2000; 10 (1), 24–30
Riteco JA, de Heij LJM, van Luijn JCF, et al. Guidelines for pharmacoeconomic research [in Dutch]. Amstelveen, The Netherlands: College of Health Care Insurance, 1999
Altman DG. Practical statistics for medical research. London: Chapham and Hall, 1991: 132–145
Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics 2000; 17 (5): 479–500
Spratt BG, Greenwood BM. Prevention of pneumococcal disease by vaccination: does serotype replacement matter? Lancet 2000; 356 (9237): 1210–1211
Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348 (18): 1737–1746
Acknowledgements
This study was performed by Jasper Bos while employed by the Netherlands Vaccine Institute. No relevant conflicts of interest exist. This study was funded by the Netherlands Vaccine Institute, Bilthoven, The Netherlands. The Netherlands Vaccine Institute is a governmental institution that, amongst others, advises on the National Vaccination Programme.
Jasper Bos was primary investigator and conducted the major share of study design, data analysis and reporting.
Hans Rümke and Loek van Alphen co-authored on the paper, provided the vaccine-specific expertise and assisted in data collection.
Robert Welte co-authored and assisted in the design of the analysis.
Lodewijk Spanjaard co-authored and assisted in data collection and verification.
Maarten Postma and Loek van Alphen supervised all stages of the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bos, J.M., Rümke, H.C., Welte, R. et al. Combination Vaccine Against Invasive Meningococcal B and Pneumococcal Infections. Pharmacoeconomics 24, 141–153 (2006). https://doi.org/10.2165/00019053-200624020-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200624020-00004