Abstract
Objective: To simulate the treatment of postmenopausal women with advanced breast cancer from second-line hormone therapy to death, and to generate estimates of the cost and effectiveness of letrozole and megestrol in order to determine the incremental cost effectiveness of letrozole, expressed as cost per life-years gained.
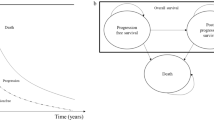
Design: A decision-analytic model, using Markov process techniques, was designed to evaluate the lifetime clinical and economic consequences of treatment with letrozole compared with standard care with megestrol. Themodel was based on clinical trial results showing a clear advantage of letrozole in terms of time to progression and duration of response.
Setting: The setting of the study was that of the UK healthcare system in 1996.
Patients and participants: A hypothetical cohort of patients, identical to the patients recruited for the AR/BC2 clinical trial,whowere postmenopausal women with advanced breast cancer who had previously failed to respond to first-line or adjuvant anti-estrogen therapy.
Interventions: The dosages of medications were 2.5 and 160 mg/day for letrozole and megestrol, respectively. The analysis covered the period from treatment initiation until death (lifetime model). Effectiveness was expressed as survival and time without progression, and the model also included all relevant economic measures.
Main outcome measures and results: Based on the model, the average survival time of the letrozole group was 2.1 years (25.3 months) versus 1.9 years (21.5 months) for the megestrol group, a gain in survival of 2.4 months (10.5%). The average time without progression, cumulatively calculated over the different treatment options, amounted to 20.2 months for letrozole and 17.8 months for megestrol, an increase of 13.7% for the former patients. The total average cost per patient for the treatment of advanced breast cancer starting from second-line hormone therapy until death was higher in the letrozole group at £7547 versus £6820 for the megestrol group (discounted at an annual rate of 5%), leading to an incremental cost-effectiveness ratio of £3588 per life-year gained (1996 values).
Conclusions: Based on the assumptions used in this model, letrozole offers a suitable alternative tomegestrol in the treatment of second-line hormone therapy.
Similar content being viewed by others
References
Levi F, La Vecchia C, Lucchini F, et al. Cancer mortality in Europe, 1990–1992. Eur J Cancer Prev 1995; 4 (5): 389–417
Jones AL, Smith IE. Medical treatment of breast cancer, 1993
Cancer Research Campaign (CRC). Factsheet no. 7. London: CRC, 1991
Butler JR, Furnival CM, Hart RF. The cost of treating breast cancer in Australia and the implications for breast cancer screening. Aust NZ J Surg 1995; 65 (7): 485–91
Elixhauser A. Cost of advanced breast cancer and the cost effectiveness of advanced breast cancer screening. Int J Technol Assess Health Care 1991; 7: 604–15
Baker MS, Kessler LG, Urban N, et al. Estimating the treatment costs of advanced breast cancer and lung cancer. Med Care 1991; 29: 40–9
Epstein RJ. Does the advanced breast cancer dollar make sense? Eur J Cancer 1992; 28: 486–91
Richards MA, Braysher S, Gregory WM, et al. Advanced breast cancer: use of resources and cost implications. Br J Cancer 1993; 67: 856–60
Hurley SF, Huggins RM, Snyder HD, et al. The cost of breast cancer recurrences. Br J Cancer 1992; 65: 449–55
De Koning HJ, van Ineveld BM, de Haes JCJM, et al. Advanced breast cancer and its prevention by screening. Br J Cancer 1992; 65: 950–5
Mouridsen HT. Systemic therapy of advanced breast cancer. Drugs 1992; 44 Suppl. 4: 17–28
Leonard RCF. Metastatic breast cancer. BMJ 1994; 309: 1501–3
Corry JF, Lonning PE. Systematic therapy in advanced breast cancer: efficacy and cost-utility. Pharmacoeconomics 1994; 5: 190–212
Rose C, Mouridsen HT. Endocrine therapy of advanced breast cancer. Acta Oncol 1988; 27: 721–8
Santen RJ, Manni A, Harvey H, et al. Endocrine treatment of breast cancer in women. Endocr Rev 1990; 11: 221–65
Dombernowsky P, Smith I, Falkson G, et al. Letrozole, a new oral aromatase inhibitor for advanced breast cancer: doubleblind randomised trial showing a dose-effect and improved efficacy and tolerability compared with megestrol acetate. J Clin Oncol 1998: 16 (2): 453–61
Nuijten MJC, Fitzimon C, Waild F, et al. Economic evaluation of letrozole in the treatment of advanced breast cancer in post-menopausal women in Canada. Value in Health. In press
Weinstein M, Fineberg H. Clinical decision analysis. London: W. B. Saunders Company, 1980
Holloway CA. Decision making under uncertainty models and choices. Englewood Cliffs (NJ): Prentice Hall Inc., 1979
Miller DK, Homan SM. Determining transition probabilities: confusion and suggestions. Med Decis Making 1994; 1: 52–8
Garcia-Giralt E, Ayme Y, Carton M, et al. Second and third line hormonotherapy in advanced post-menopausal breast cancer: a multicenter randomized trial comparing medroxyprogesterone acetate with aminoglutethimide in patients who have become resistant to tamoxifen. Breast Cancer Res Treat 1992; 24: 139–45
Brufman G, Isacson R, Haim N, et al. Megestrol acetate in advanced breast carcinoma after failure to tamoxifen and/or aminoglutethimide. Oncology 1994; 51 (3): 258–61
Iveson TJ, Ahern J, Smith TE. Response to third-line endocrine treatment for advanced breast cancer. Eur J Cancer 1993; 29A (4): 572–4
Ingle JN, Johnson PA, Suman VJ, et al. A randomised phase II trial of two dosage levels of letrozole as third-line hormonal therapy for women with metastatic breast carcinoma. Cancer 1997; 80 (2): 218–24
Alonso MC, Tabernero JM, Ojeda B, et al. Aphase III randomized trial of cyclophosphamide, mitoxantrone, and 5-fluorouracil (CNF) versus cyclophosphamide, adriamycin, and 5-fluorouracil (CAF) in patients with metastatic breast cancer. Breast Cancer Res Treat 1995; 34 (1): 15–24
Fraser SCA, Dobbs HJ, Ebbs SR, et al. Combination or mild single agent chemotherapy for advanced breast cancer? CMF vs epirubicin measuring quality of life. Br J Cancer 1993; 67: 402–6
Viladiu P, Alonso MC, Avella A, et al. Chemotherapy versus chemotherapy plus hormonotherapy in postmenopausal advanced breast cancer patients. Cancer 1985; 56: 2745–50
Mouridsen HT, Rose C, Engelsmann E, et al. Combined cytotoxic and endocrine therapy in postmenopausal patients with advanced breast cancer. J Steroid Biochem 1985; 23 (6B): 1141–6
Hoogstraten B, Gad-el-Mawla N, Maloney TR, et al. Combined modality therapy for first recurrence of breast cancer. Cancer 1984; 54: 2248–56
Gerritsen M, Wagener DJ, Schade RW, Breast Cancer Study Group, et al. Palliative chemotherapy with CMF after the same adjuvant regimen for breast cancer. Neth J Med 1995; 46 (3): 131–5
Gregory WM, Smith P, Richards MA, et al. Chemotherapy of advanced breast cancer: outcome and prognostic factors. Br J Cancer 1993; 68 (5): 988–95
Petru E, Schmähl. On the relevance of ’second-line’ cytotoxic chemotherapy in patients with metastatic breast cancer resistant to standard combinations. Wien Klin Wochenschr 1986; 23: 790–7
Fink A, Kosecoff J, Chassin M, et al. Consensusmethods: characteristics and guidelines for use. Am J Public Health 1984; 9: 979–83
Jariath N, Weinstein J. The Delphi methodology (Pt 2): a useful administrative approach. Cand J Nurs Adm 1994; 7 (4): 7–20
Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995; 311: 376–80
Hutton J, Brown R, Borowitz M, et al. A new decision model for cost-utility comparisons of chemotherapy in recurrent metastatic breast cancer. Pharmacoeconomics 1996; 9 Suppl. 2: 8–22
Author information
Authors and Affiliations
Corresponding author
Additional information
Work conducted while an employee at Benefit B. V., Leiden, The Netherlands
Rights and permissions
About this article
Cite this article
Nuijten, M., Meester, L., Waibel, F. et al. Cost Effectiveness of Letrozole in the Treatment of Advanced Breast Cancer in Postmenopausal Women in the UK. Pharmacoeconomics 16, 379–397 (1999). https://doi.org/10.2165/00019053-199916040-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-199916040-00006