Abstract
Background and Objective
Trastuzumab was introduced in Sweden in 2000 for treatment of HER2-positive metastatic breast cancer (MBC) and later expanded to early breast cancer (EBC). The potential value of this innovative therapy was explored in economic evaluations; however, the extent to which these benefits were realised remains unknown. This study aims to estimate the lifecycle value of trastuzumab by combining randomised trial data with Swedish routine-care data.
Methods
Trastuzumab impact on costs and health outcomes was estimated with Markov models for MBC and EBC. Model inputs included progression/recurrence and breast cancer-related mortality data from international randomised clinical trials, and Sweden-specific non-breast cancer-related mortality, numbers treated, and costs and utilities based on data from National Registries and literature. Model predictions were validated by observed survival rates from the National Breast Cancer Registry.
Results
From 2000 to 2021, 3936 and 11,134 patients with HER2-positive MBC and EBC, respectively, were treated with trastuzumab, resulting in 25,844 life years and 13,436 per quality-adjusted life years (QALY) gained. Cost per QALY gained was lower in EBC (Swedish krona [SEK] 285,000/QALY) than MBC (SEK 554,000/QALY). The net-monetary value delivered (excluding drug costs) was SEK 13.714 billion, and society retained 62% of this. The modelled survival in trastuzumab-treated patients with EBC matched closely with actually observed survival in registry data.
Conclusion
Trastuzumab provided substantial population-level health benefits for patients and society, with favourable cost effectiveness in MBC and EBC. There is some uncertainty around the magnitude of these benefits, mainly due to missing data on health outcomes and number of treated patients with MBC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Swedish society retained two-thirds of the value generated by trastuzumab and the remainder was allocated to the innovator. |
Data from the registry reveal that the foregone benefit due to undertreatment, especially in women aged ≥ 70 years, is substantive. |
Leveraging real-world evidence along the lifecycle of innovative therapies, to evaluate value derived from therapeutic and other types of benefits actually delivered, may support better allocation decisions based on a reward mechanism that adapts to incorporate new information generated once the treatment is in use. |
1 Introduction
Decision-making in the assessment of innovative health technologies conducted by regulators, reimbursement authorities, and endorsement bodies is based on limited evidence of the technology's benefits, risks, and costs. As such, measures to offset the uncertainty have been developed, which impact their adoption. When evidence generated after initial approval demonstrates that the new technology is less safe or efficacious, or is less cost-effective than originally thought, their marketing authorisation can be revoked [1], they can be excluded from the national or local formulary, and/or their coverage can be restricted or withdrawn. Such was the case of olaratumab for soft-tissue sarcoma, bevacizumab for breast cancer [2], or everolimus for pancreatic neuroendocrine tumours [3]. However, in other cases, post-marketing authorisation evidence settles the uncertainty in favour of the benefits of a medical innovation. This was the case of trastuzumab in the treatment of patients with breast cancer (BC) overexpressing the human epidermal growth factor receptor 2 (HER2) gene.
Trastuzumab was first approved in 1998 by the United States (US) Food and Drug Administration (FDA) for the treatment of metastatic disease, which was supported by the results from two pivotal trials that demonstrated the efficacy and safety of trastuzumab based on surrogate endpoints [4]. Subsequent studies demonstrated that progression-free survival (PFS) was significantly longer when adding trastuzumab to chemotherapy (7.6 vs 4.6 months with chemotherapy alone) as was median overall survival (OS) (25.4 vs 20.3 months) [5, 6]. In 2006, trastuzumab was approved for use in the adjuvant setting for patients diagnosed in early stages. Several large clinical trials demonstrated improved OS and PFS, as well as a reduction in relapses. For example, Slamon et al found that 5-year OS was 87% among patients receiving docetaxel, 92% among those receiving docetaxel plus trastuzumab, and 91% among those receiving docetaxel and carboplatin plus trastuzumab. Additionally, the trial demonstrated a significant gain in relapse as 5-year disease-free survival was 75%, 84%, and 81%, respectively [7]. These findings were confirmed in other trials [8,9,10] and trastuzumab became a case study for the research on value of innovation.
Garrison and Veenstra [11] developed a dynamic model to assess the clinical and economic value of trastuzumab over its entire lifecycle, from first approval in the USA in 1998 until 2016 (10 years after its introduction in the adjuvant setting). They concluded that the USA would gain 432,547 quality-adjusted life years (QALY), which was worth $21.6 billion to $64.9 billion, depending on the willingness-to-pay threshold. These gains would represent a societal net economic value between $6.2 billion to $49.5 billion. Similarly, in 2013, Lundqvist et al appraised the overall societal value of this and other innovative targeted drugs in Sweden as part of a research programme launched by SNS. This study predicted an aggregate, population-level health gain of 5.2 billion Swedish krona [SEK] (in 2012 SEK) as a result of treatment with trastuzumab in both indications, which greatly offsets the total drug cost of SEK2.0 billion [12].
Both studies were based on trial data, extrapolation of long-term effects on survival, and projections of disease incidence and drug uptake in both indications. Today, 20 years after the introduction of trastuzumab in Sweden, sufficient evidence has been accrued of the actual use, total budget spent in the acquisition of the drug, and benefits of trastuzumab in routine clinical practice. Thus, an update of this research is warranted to validate the assumptions and findings in trastuzumab initial assessment and, most importantly, to understand the extent to which coverage, reimbursement, and treatment decisions over these years, have maximised the health and economic benefits that this innovation had to offer.
The goal of this study was to assess the total value of trastuzumab over its life cycle, by aggregating the lifetime clinical and economic benefits derived from its actual use in the treatment of patients with HER2+ BC between 2000 and 2021 in Sweden, in terms of total life-years gained (LYG), QALYs gained, productivity gains, and health care cost savings.
2 Methods
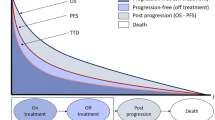
The economic evaluation, performed from a modified societal perspective (following updated guidelines from the Swedish Dental and Pharmaceutical Benefits Agency [Tandvårds- och läkemedelsförmånsverket—TLV]), synthesises the outcomes of two three-state Markov models comparing trastuzumab-containing regimens with standard of care (SoC) at time of introduction (see Fig. 1 for a graphical representation).
In the following sections, we describe the model structure and inputs. Real-world data from Sweden were used when available, from public sources or from the literature, and only supplemented with trial data for the efficacy parameters. A summary of all model inputs is presented in Tables 1 and 2.
2.1 Metastatic Disease
The model for advanced or metastatic breast cancer (MBC) simulates the treatment and outcomes of patients diagnosed in stages IIIB+IV. All patients entered the model in the pre-progression state (PPD) and transitioned to progressive disease (PD) and/or death (final and all-absorbing state). The model was run for 120 monthly cycles (10 years) and evaluated separately by ten 5-year age groups (< 40 years, assumed starting age 35 years; 40 to 45 years, starting age 42 years; 45 to 50 years, starting age 47 years; …; > 80 years, assumed starting age 82 years). Transition probabilities were estimated based on data on median PFS and median OS from the literature [6], to parametrise the respective exponential survival functions. The annual probabilities of exiting the PPD state and of entering the death state were estimated for each treatment arm. Then, the probability of remaining in the PD state was calculated as one minus the probability of being in the PPD or death states. Transition probabilities used in the MBC model can be found in Supplementary Online Table 1.
2.2 Adjuvant/Neoadjuvant Treatment of Early Disease
The model for patients with early disease diagnosed in stages I to IIIA (early breast cancer [EBC]) simulates the adjuvant or neoadjuvant treatment and outcomes of patients who transition from remission (the initial state) to recurrence and to death (final and all-absorbing state). The model was run for 50 yearly cycles and evaluated separately by age group in the same way as the MBC model. Patients who experience a recurrence remain in this state until the end of the simulation or they transit to the death state.
In the EBC model, non-cancer-related mortality is modelled as a transition from remission to death. Actual survival data for Sweden were used, by year of age. This was calculated as the number of deaths within each age group during the year 2020 divided by the total population in each age group at the beginning of 2020 (data from Statistics Sweden [SCB]) [13]. The yearly rate of recurrence (transition from remission to recurrence) was obtained from a review and meta-analysis of seven randomised clinical trials followed for up to 10 years after treatment initiation [10]. Post-recurrence mortality (transition from recurrence to death) was calibrated in the model to yield the observed OS in the clinical trial meta-analysis. All transition probabilities used in this model can be found in Supplementary Online Table 2 and background mortality in Supplementary Online Table 3.
2.3 Costs
The costs of care in different BC disease states in Sweden were retrieved from a retrospective database study published by Lidgren et al [14], and inflated to 2021 prices using the health care price index [15]. Costs, including direct medical costs, informal care, and productivity are presented separately for patients with EBC in remission, after recurrence, and for patients with metastatic disease. Indirect costs, or productivity losses, were presented separately by age group (<50, 50 to 64 years), while no productivity costs were added for patients aged ≥65 years. For patients who died before the age of 65 years, productivity loss was assumed to be the same as that for patients with first-year recurrence.
Total trastuzumab acquisition cost was obtained from national sales data for the years 2000 to 2021, provided by the Swedish eHealth agency [16], inflated to 2021 prices using the SCB consumer price index [17]. Additionally, the per-patient cost associated with the administration of trastuzumab treatment was estimated based on results of a micro-costing study conducted in Sweden by Olofsson et al [18], assuming that the distribution of patients who received trastuzumab intravenously (IV) versus subcutaneously (SC) was 66% versus 34%. Direct costs included nurse time, medical supplies, pre-medication, and transportation. Only production lost by the patient for the administration of trastuzumab was considered to estimate the indirect costs associated with the intervention, to avoid double counting. Cost of informal care included time off work and leisure time lost by the patients’ accompanying kin. These costs per cycle were multiplied by the corresponding number of expected cycles in MBC and EBC settings to obtain the per-patient cost of administration (more details on the assumptions can be found in Tables 1 and 2).
All future costs were discounted to present value at 3% annually.
2.4 Utilities
Utility values were retrieved from a naturalistic cross-sectional study conducted by Lidgren et al to assess health-related quality of life of patients with BC in different health states in Sweden [19]. Future QALYs were discounted at 3%.
2.5 Numbers Treated
The total number of patients with EBC treated with trastuzumab, per year, age group, and calendar year between 2008 and 2020, was obtained from the National Quality Registry for Breast Cancer (NKBC). These counts were then supplemented with national aggregated sales data provided by eHälsomyndigheten to estimate numbers of patients with EBC treated in 2005, 2006, 2007, and 2021, as well as the number of patients with MBC treated in 2000 through 2021 as follows:
-
Metastatic breast cancer 2000 to 2004: In this period, trastuzumab was used only in MBC, so the number of patients was estimated by dividing the national total sales by the estimated cost per patient treated in MBC.
-
Metastatic breast cancer 2008 to 2020: The numbers treated were estimated by subtracting the cost of treatment in EBC by year and age group from the yearly sales data and dividing by the cost per patient with MBC. The age distribution for patients with MBC was assumed to follow that of patients with EBC.
-
Metastatic breast cancer 2005 to 2008: The numbers treated were interpolated linearly between the known values for 2004 and 2008.
-
Early breast cancer 2005 to 2008: The numbers treated in EBC were estimated by subtracting the cost of treatment in MBC from the yearly sales data, and dividing by the cost per patient with EBC.
-
Early breast cancer and MBC 2021: The numbers treated were assumed to be the same as for 2020.
-
In 2019 and 2020, the significant drop in total sales (42% and 61%, respectively) was likely due to trastuzumab patent expiration in 2018. Thus, we assumed that the price erosion was 50% and 60%, respectively.
2.6 Sensitivity Analyses
All model inputs have been derived from actual population-based data, except for the efficacy parameters that reflect findings from randomised clinical trials. Thus, this is the single source of uncertainty that remains to be incorporated in the analysis. Two types of sensitivity analyses were conducted:
-
Scenario analysis to understand the impact of uncertainty around the effectiveness of trastuzumab on the results: We ran a ‘Best-case Scenario’ and a ‘Worst-case Scenario’ by modifying the effectiveness parameters in the trastuzumab arms. For the EBC model, we used the upper- and lower-bound of the 95% confidence interval (CI) of the relative risk (RR) of recurrence estimated in the Early Breast Cancer Trialists' Collaborative Group meta-analysis [10] to increase/decrease trastuzumab effectiveness (RR 0.643 [95% CI: 0.597, 0.692]). For the base case of the MBC model, we used per-protocol estimates of median survival from a clinical trial in which 57% of patients in the placebo arm crossed-over to the active arm, so this article did not provide data on CIs. Thus, we used the upper and lower bound of the CI of the hazard ratio (HR) estimated in a Cochrane meta-analysis conducted in 2014 in this indication [20] to increase/decrease OS in trastuzumab-treated patients (HR 0.82 [95% CI: 0.71, 0.94]).
-
Validation of modelled survival against real-world estimates: The modelled OS in the EBC model was compared to the observed 5-year and 10-year OS for HER2+ patients published by the NKBC in each age group.
-
Alternative perspectives: The core scenario is based on a modified societal perspective that incorporates productivity losses, assuming all patients retire at 65 years of age. To assess the impact of the perspective on the monetary value of health and economic benefits as well as the distribution of welfare surplus between the innovator and the Swedish society, two additional sets of results are presented:
-
Healthcare perspective: Incorporates only direct medical costs and excludes informal care and productivity losses
-
Full societal perspective: Incorporates costs in added years of life by deducting production from consumption, for each subgroup of treated patients defined by the age ranges based on consumption costs published in Ekman 2002, and inflated to 2021 SEK based on the Swedish Consumer Price Index.
-
3 Results
Key study results can be found in Table 3. We estimated that, between 2000 and 2021, 15,070 patients were treated with trastuzumab in Sweden (3936 with MBC and 11,134 with EBC). Supplementary Online Table 4 shows trastuzumab fast uptake in the adjuvant/neoadjuvant setting—by 2008 it had already become the standard of care. Based on these data on actual treatment from NKBC, between 2008 and 2020, 77% of patients diagnosed with HER2+ EBC received trastuzumab (9539 out of 12,345), with the lowest registered in 2008 (62%) and the highest in 2012 (82%). Figure 2 presents the proportion of patients diagnosed with HER2+ EBC treated with trastuzumab between 2008 and 2020 in Sweden, stratified by region and age groups. Trastuzumab treatment was administered to 9 in every 10 patients diagnosed aged < 70 years, but only one in two patients aged ≥ 70 years. The differences were most pronounced in the West and Uppsala/Orebro Regions.
A total of 25,844 life years were gained thanks to the use of trastuzumab, almost three-quarters of which came from treating the disease at earlier stages. We estimate that, after adjusting for the impact of the disease and its treatment on the patients’ quality of life, Swedish society gained a total of 13,436 QALYs.
Sensitivity analyses demonstrate that, while the findings are consistent across scenarios, there is a difference in magnitude as the health benefits in terms of LYGs and QALYs derived from the use of trastuzumab would be 28% and 26% lower in the worst-case effectiveness scenario or 26% and 23% higher in the best-case effectiveness scenario, respectively.
Figure 3 shows the modelled OS in the EBC model, compared with the observed 5-year and 10-year OS in the NKBC (among HER2+ patients) for all age groups. The model survival function predicts closely both landmark OS estimates published by the registry in all age groups.
Table 3 also shows the estimated economic impact of the actual use of trastuzumab between 2000 and 2021 in Sweden. The Swedish health care system paid SEK5.2 billion (2021 SEK equivalent to 2021 US$606 million) for the drug and SEK163 million ($19 million) for its administration, in addition to SEK1.5 billion ($176 million) for the overall management of these patients. The total cost of informal care, amounting to SEK263 million ($30 million), was split differently between indications. Supporting patients during treatment administration took more time for caregivers of patients with early than metastatic disease (SEK118 vs SEK32 million [$13.7 vs $3.7 million]). Conversely, supporting patients with all other disease-related activities took more time for the care of patients with MBC than EBC (SEK76 vs SEK37 million [$8.8 vs $4.3 million). Productivity losses due to the administration of trastuzumab were also higher in the adjuvant setting than in metastatic (SEK25 vs SEK6 million [$2.9 million vs $721,000]), but the societal gains from indirect costs averted more than offset those losses. The use of trastuzumab saved SEK2.24 billion ($261 million) in indirect costs. Overall, the total cost born by the Swedish society in the care of these patients over the past 21 years was SEK4.92 billion ($573 million), and a slightly larger share of these costs were spent in patients presenting with early disease (SEK2.66 billion [$311 million] compared with SEK2.26 billion [$263 million]).
Based on our models, we estimate that the lifecycle monetary value of health benefits gained from the use of trastuzumab in Sweden over the past 21 years was SEK13.4 billion ($1.57 billion), assuming a SEK1 million willingness-to-pay threshold based on the inflation-adjusted valuation published by Hultkranz and Svensson [21]. After deducting total direct and indirect costs considered from a modified societal perspective, the net monetary value delivered by trastuzumab was SEK8.52 billion ($992 million). Excluding drug costs, SEK13,7 billion ($1.6 billion) in welfare surplus was allocated 38%–62% to the producer (innovator) and consumer (Swedish society), respectively.
Altering the cost-accounting perspective does impact the net monetary value of trastuzumab. While adopting a narrow health care perspective would represent a 28% increase in the cost per QALY, Swedish society still retains a majority of the surplus (56%). If instead, costs are estimated based on TLV’s old method for the full societal perspective, incorporating cost per years in added life, the net monetary value delivered by trastuzumab would drop by 41%. While the mean cost per QALY (SEK616,328 [$71,821]) remains under the willingness-to-pay threshold, the welfare surplus is split evenly between the innovator and society (Table 4).
4 Discussion
The health benefits derived from the use of trastuzumab have been considerable. According to information published by the Swedish government through their Health and Welfare Statistical Database, 23,070 people died of cancer in 2021 (Cause of Death Statistics, Number of deaths, C00-D48 Neoplasms, Entire Sweden, Age: 0–95+, both sexes, 2021). If there were a health intervention that could have extended the lives of all these people by one year, it would have still delivered fewer LYG than trastuzumab (25,844).
Our study investigated the direct contribution of trastuzumab on survival over a 21-year period, as compared with SoC, based on clinical trials conducted at the start of that period. A closer examination of the evolution of SoC in this indication reveals that it has remained rather stable during this time (2000–2021). Standard of care, then and now, includes combinations with chemotherapy such as taxanes (docetaxel or paclitaxel), capecitabine or platinum compounds (cis- or carboplatin) and/or hormonal therapies such as tamoxifen and aromatase inhibitors (anastrozole, exemestane, and letrozole), these only for patients with hormone sensitive, HER2-positive disease. These drugs were all well established as part of the treatment armamentarium in both early and metastatic breast cancer by the time trastuzumab was added as the backbone medicinal therapy for HER2-postive patients. As for new therapies, some of these patients also benefitted from treatment with lapatinib, pertuzumab, trastuzumab emtansine, neratinib, trastuzumab deruxtecan, and tucatinib. These innovative treatments introduced are normally used either in combination with or as second line following trastuzumab. Thus, those patients treated sequentially, who were diagnosed before the newer drug was available, have also gained from the real option value provided by trastuzumab to live long enough to benefit from the next innovation. Several studies have borrowed this concept and corresponding methods from financial theory to estimate the size of these gains [22,23,24], although we chose to provide a conservative estimate.
Overall, our findings are aligned with those of Garrison and Veenstra [11] and Adam Lundkvist et al. [12], but there are differences in the inputs and magnitude of the effect that are worth discussing. Our study included real-world estimates of cost of care for the underlying disease, cost of treatment acquisition and administration, indirect costs, and informal care. Neither of the aforementioned studies considered the overall direct medical cost (beyond those associated with the intervention) or cost of informal care, and Garrison and Veenstra did not consider productivity losses, while Lundkvist et al used a different approach also incorporating the value of consumption. Despite these differences, the estimated QALYs gained per patient were similar to those in our study (0.90 in Garrison and Veenstra, 0.78 to 1.11 in Lundkvist et al, and 0.89 in our study). Our estimates in two sensitivity analyses (incorporating the cost of added years of life and shifting fully to the health care perspective) demonstrate that the value Swedish society derived from the use of trastuzumab remains significantly higher than the minimum threshold and larger than the share retained by the innovator. Of note, the change in magnitude highlights the fact that much of the value was realised outside of the health care system.
Among HTA bodies and pricing and reimbursement agencies, the choice of perspective for economic evaluation is a controversial issue, and guidelines and recommendations have been changed over time. As an example, the ‘full societal perspective’ incorporating net consumption in added life years was previously recommended by TLV but, in recent years, it has been abandoned. In TLV’s report issued in 2021 “How should we assess and pay? Health-economic assessments and payment models for precision medicines and ATMPs”, the following explanation was offered:
“Providing life-saving treatments to patient groups in retirement ages would then be considered more expensive than doing so for a patient group of working age. It would also be considered more expensive to provide life-prolonging treatments to groups with disabilities or others who are not expected to work. … If calculation and prioritisation were to be based on such grounds, then groups that do not work and which will not start working after treatment risk having poorer access to both quality-of-life improvements and life-prolonging treatments than others. After examining the ethical appropriateness of this approach, TLV found that it was incompatible with the ethical platform’s human-value principle. Consequently, TLV changed its general guidelines for health-economic assessments …[and] now applies a narrower societal perspective than before its change in approach. However, TLV does not adopt a strict health care perspective. Costs and savings outside of health care can also be taken into account.”
We consider that the base case (modified societal perspective) is a balanced approach because it does not incorporate the negative impact of costs for added years of life in the older population, but it does not account for the productivity losses among women who continue to work beyond the age of 65 years. Based on data extracted from Statistics Sweden, 27% of women aged between 65 and 69 years and 10% of those aged 70 to 74, were still employed in 2022 [25]. Furthermore, in the base-case proposed, we have also included costs for informal care, which is one of the components of the key consumption items in the estimation of cost of added years of life.
Thus, our results are likely underestimating the magnitude of the benefit derived by society because they do not tally real option value or the value of incremental innovations, and because the significant price erosion following trastuzumab patent expiration in 2018, was and will continue to be larger than accounted for in our model inputs. It is worth mentioning that the emergence of biosimilars as of that moment, has further reduced the share of the surplus claimed by the innovator. According to the data provided by the NKBC, one in every four HER2+ patients diagnosed with EBC was not treated with trastuzumab. Since therapeutic decisions are patient-centric, the lack of information on the reason for not using trastuzumab in adjuvant setting for each of those patients, prevents us from making any inference. Alhough, the marked difference in age distribution (14% of younger vs 48% of older patients did not receive treatment) indicates that the under-treatment may be driven by stopping rules rather than patient-specific clinical decisions. If this were the case, this treatment under-usage is likely associated with foregone patient and societal benefits in terms of survival and QALYs lost.
An important limitation of this study is that certain model parameters (such as post-recurrence or post-progression mortality) had to be derived from old clinical trial data calibrated based on a number of assumptions. Equally, the estimation of the number of patients actually treated in the metastatic setting was also based on assumptions. Even though it has been more than 20 years since trastuzumab was introduced in Sweden, and the country has some of the world’s best registries in routine care, some uncertainty still remains regarding the usage and impact of trastuzumab. Information of this nature is of crucial importance to assess the value of new health technologies in routine care; especially now when numerous cancer therapies are approved, through early access schemes, based on data from single-arm trials and the commitment to continue generating evidence post-authorisation.
5 Conclusion
Trastuzumab has provided substantial population-level health benefits for patients with HER2+ BC in all settings and stages over the past 21 years in Sweden. The value created was not only enjoyed by the patients who lived longer and experienced fewer relapses, but also by the health care system and society as a whole. In fact, the weight of accounting for averted productivity losses is such, that it could shift the conclusions. Taking these gains into consideration, we conclude that society claimed almost two-thirds of the surplus. Not accounting for these gains, the surplus is almost evenly split. The debate around cost-accounting perspective is far from being resolved; but we hope that this study contributes to the understanding of the dynamics at play in lifecycle evaluations of innovative health interventions. The societal gain was realised and, even if only as a positive externality to investments in the health care system, it should not be overlooked.
Our study confirmed that health and economic gains have greatly surpassed those anticipated at product launch at the turn of the century and, while the evaluation methods sufficed to predict this success story, it was impossible to commensurate back then what we now know. Yet, due to variable access to treatment across health care regions and the trend to undertreat patients aged ≥70 years, the full potential of this drug has not been realised. The reasons behind these differences remain elusive. Possible interpretations are disparities in testing strategies, variations in the interpretation of clinical data, or differing rationale for budget-allocation decisions. In any case, the evidence suggests that health care provision is not consistent throughout the country, at least for women diagnosed with HER2+ BC. The creation of national quality registries several decades ago, aimed to shed light on these types of divergences and foster standardisation by disseminating best practices. The NKBC collects an impressive amount of data of the highest quality although, the data routinely and systematically collected is deficient to fully inform these type of research questions, and to eliminate uncertainty on the value actually delivered by innovative health interventions, even today with hindsight of >20 years.
References
Vandegrift D, Datta A. Prescription drug expenditures in the united states: the effects of obesity, demographics, and new pharmaceutical products. South Econ J. 2006;73(2):515–29.
Vitry A, Nguyen T, Entwistle V, Roughead E. Regulatory withdrawal of medicines marketed with uncertain benefits: the bevacizumab case study. J Pharm Policy Pract. 2015;8:25.
Aggarwal A, Fojo T, Chamberlain C, Davis C, Sullivan R. Do patient access schemes for high-cost cancer drugs deliver value to society?-lessons from the NHS Cancer Drugs Fund. Ann Oncol. 2017;28(8):1738–50.
Smith IE. Efficacy and safety of Herceptin in women with metastatic breast cancer: results from pivotal clinical studies. Anticancer Drugs. 2001;12(Suppl 4):S3-10.
Eiermann W, International Herceptin Study G. Trastuzumab combined with chemotherapy for the treatment of HER2-positive metastatic breast cancer: pivotal trial data. Ann Oncol. 2001;12:S57-62.
Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23(19):4265–74.
Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–83.
Hurley J, Doliny P, Reis I, et al. Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J Clin Oncol. 2006;24(12):1831–8.
Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84.
Early Breast Cancer Trialists' Collaborative group (EBCTCG). Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021;22(8):1139-1150.
Garrison LP Jr, Veenstra DL. The economic value of innovative treatments over the product life cycle: the case of targeted trastuzumab therapy for breast cancer. Value Health. 2009;12(8):1118–23.
Adam Lundqvist NW, Ulf-G Gerdtham, Ulf Persson, Katarina Steen Carlsson. Målriktad behandling av bröstcancer Stockholm, Sweden: SNS;2013.
Statistics Sweden. Mortality statistics published by SCB. https://www.statistikdatabasen.scb.se/pxweb/sv/ssd/START__BE__BE0101__BE0101I/DodaFodelsearK/. Accessed 1 Nov 2022.
Lidgren M, Wilking N, Jonsson B, Rehnberg C. Resource use and costs associated with different states of breast cancer. Int J Technol Assess Health Care. 2007;23(2):223–31.
Sveriges Kommuner och Regioner. Healthcare price index (Vårdprisindex-VPI). https://skr.se/skr/ekonomijuridik/ekonomi/budgetochplanering/prisindex/vpi.31954.html. Accessed 8 Mar 2022.
E-hälsomyndigheten. Drug Sales Statistics (Försäljningsstatistiken). https://www.ehalsomyndigheten.se/statistik-och-lakemedelsforsaljning/bestalla-statistik/. Accessed 8 Mar 2022.
Statistics Sweden. Consumer price index (KPI). https://www.statistikdatabasen.scb.se/pxweb/sv/ssd/START__PR__PR0101__PR0101A/. Accessed 8 Mar 2022.
Olofsson S, Norrlid H, Karlsson E, Wilking U, Ragnarson TG. Societal cost of subcutaneous and intravenous trastuzumab for HER2-positive breast cancer—An observational study prospectively recording resource utilization in a Swedish healthcare setting. Breast. 2016;29:140–6.
Lidgren M, Wilking N, Jonsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res. 2007;16(6):1073–81.
Balduzzi S, Mantarro S, Guarneri V, et al. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database of Systematic Rev. 2014;2014(6) (no pagination).
Hultkrantz L, Svensson M. Värdet av liv. Ekonomisk debatt. 2008;2:5–16.
Cook JP, Golec JH, Vernon JA, Pink GH. Real option value and path dependence in oncology innovation. Int J Econ Bus. 2011;18(2):225–38.
Li M, Basu A, Bennette CS, Veenstra DL, Garrison LP. Do cancer treatments have option value? Real-world evidence from metastatic melanoma. Health Econ. 2019;28(7):855–67.
Garrison LP, Towse A. A strategy to support efficient development and use of innovations in personalized medicine and precision medicine. J Manag Care Spec Pharm. 2019;25(10):1082–7.
Labour market status by region, sex, age and region of birth. Preliminary statistics. Year 2020–2022. Statistics Sweden. https://www. stati stikd ataba sen. scb. se/ pxweb/ en/ ssd/ START__ AM__ AM0210__ AM021 0A/ ArbSt atusAr/ table/ table ViewL ayout1/. Accessed 24 May 2023.
Acknowledgements
The authors would like to thank the authors of the SNS Report “Målriktad behandling av bröstcancer”, Adam Lundkvist, Ulf-G Gerdtham, Ulf Persson, and Katarina Steen Carlsson. This work was based in part on that report.
Funding
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors’ contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work, and have given final approval to the version to be published. All authors contributed to the conceptualization of the study, data curation, investigation, reviewing and editing of the manuscript. NJ and LJ contributed to the formal analyses, and project administration. LJ designed the methodology and model. NJ wrote the original draft. LJ and NW contributed to the supervision and validation of findings.
Funding
No funding was received to conduct this study.
Data availability
The datasets generated and/or analysed during this study were obtained from the Swedish National Quality Register for Breast Cancer (NKBC) and eHälsomyndigheten. Summary data from the former can be found here: https://statistik.incanet.se/brostcancer/. Patient-level data from the former and product-specific sales data from the latter are only available upon application and approval of the study-specific request.
Code availability
Analyses of the individual-patient level data were performed in R, the models were built in Excel
Ethics Approval
The study was approved by the Swedish Ethical Review Authority (Etikprövningsmyndigheten) Dnr 2021-06709-01 and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Competing interests
Nahila Justo is employed by Evidera, which provides consulting and other research services to pharmaceutical, medical devices, and related organisations. In her salaried position, she works with a variety of companies and organisations, and is precluded from receiving payment or honoraria directly from these organisations for services rendered. The work herein was not conducted as part of her employment. Nils Wilking reports personal fees from AstraZeneca, Bayer, Daichii Sankyo, Incyte, Jansen, MSD, Novartis, Pierre Fabre and Oasmia for participation in advisory boards and educational activities. Linus Jönsson has no competing interests to declare.
Consent to participate
Not applicable
Consent for publication
Not applicable
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Justo, N., Wilking, N. & Jönsson, L. Estimating the Product Lifecycle Value of Trastuzumab Based on Registry Data in Sweden. Clin Drug Investig 43, 529–540 (2023). https://doi.org/10.1007/s40261-023-01279-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-023-01279-2