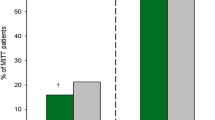
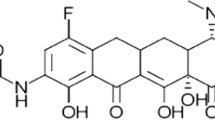
Abstract
Synopsis
Imipenem/cilastatin possesses a very broad spectrum of antibacterial activity that encompasses the range of Gram—negative and Gram—positive aerobes and anaerobes usually associated with intra—abdominal and other polymicrobial infections. Its therapeutic efficacy is comparable to that of aminoglycoside/antianaerobe combination regimens, and the most commonly reported adverse effects are similar to those of other β—lactam antibacterials and are generally of a non—serious nature.
The acquisition cost of imipenem/cilastatin is generally greater than that of aminoglycoside/ antianaerobe combination regimens, but treatment with the latter incurs the additional costs of multiple intravenous administration, aminoglycoside pharmacokinetic and other monitoring, and possible nephrotoxicity and ototoxicity. The available pharmacoeconomic studies show a trend towards lower total treatment costs with imipenem/cilastatin compared with gentamicin plus c/indamycin. Results from other sources suggest that imipenem/cilastatin may achieve forther cost savings through reduced duration of hospitalisation. Although forther study is required to confirm these trends, it appears that the total treatment cost of imipenem/cilastatin does not exceed that of usual combination therapy and the risk of aminoglycoside—induced toxicity is avoided.
Disease Considerations
Most intra—abdominal infections are polymicrobial, and Bacteroides fragilis and Escherichia coli are the most frequently isolated aerobes and anaerobes, respectively. Severe infection carries the risk of sepsis—induced multiple organ failure and requires early treatment. The major factors contributing toward the total direct cost of treatment include diagnostic procedures, surgery, hospitalisation, resuscitation and nutritional support, and adjunctive antibacterial therapy. The latter requires a broad spectrum regimen suitable for empirical administration.
Intravenous administration of an aminoglycoside in combination with an antianaerobe, such as metronidazole or clindamycin, is a widely used and effective treatment. However, administration costs are greater for a multiple versus single agent intravenous regimen, and aminoglycoside treatment increases the cost of pharmacokinetic monitoring and may also result in costs due to nephrotoxicity or ototoxicity. Thus, it is appropriate to evaluate alternative antibacterial regimens, particularly from a pharmacoeconomic standpoint.
Pharmacoeconomic Benefits and Costs
The economic potential ofimipenem/cilastatin hinges on its ability to be as effective as standard combination regimens but to incur lower overall treatment costs. Imipenem/cilastatin possesses an extremely broad spectrum of antibacterial activity that covers the range of Gram—negative and Gram—positive aerobes and anaerobes usually associated with intra—abdominal infections. In comparative trials involving patients with intra—abdominal or other polymicrobial infections intravenous imipenem/cilastatin (usually 0.5g every 6 hours) exhibited comparable efficacy to various combination regimens. The latter most frequently comprised clindamycin plus an aminoglycoside such as gentamicin or tobramycin.
In general, preparation and administration costs of intravenous monotherapy are lower than those of combination treatment. Imipenem/cilastatin eliminates the costs of pharmacokinetic monitoring as well as the risk of additional costs incurred from aminoglycoside toxicity. There is also some evidence that there may be fewer costs due to treatment failure with imipenem/ cilastatin. A few studies have reported a reduction in hospital length of stay with imipenem/ cilastatin treatment but the available evidence is insufficient to be considered conclusive.
The acquisition cost of daily therapy with imipenem/cilastatin is generally greater than that of a typical combination regimen. Costs due to adverse events are not expected to be significant with imipenem/cilastatin, although on rare occasions the cost of seizure will be an important consideration.
Pharmacoeconomic Analysis
The available comparative pharmacoeconomic data are limited to 3 cost—minimisation studies. While there appears to be a trend toward lower total treatment costs with imipenem/cilastatin compared with gentamicin plus c1indamycin, there is insufficient evidence to demonstrate a definite cost advantage favouring the former treatment.
Formulary decisions on the choice of antibacterial agent for the treatment of intra—abdominal infection should be based on institution—specific data. When this is not available, and in view of the above findings, the deciding factor might well be the risk of aminoglycoside—induced toxicity.
Similar content being viewed by others
References
Bartlett JG, Sullivan-Sigler N, Louie TJ, Gorbach SL. Anaerobes survive in clinical specimens despite delayed processing. Journal of Clinical Microbiology 3: 133–136, 1976
Birnbaum J, Kahan FM, Kropp H, Mac Donald JS. Carbapenems, a new class of beta—lactam antibiotics. American Journal of Medicine 78 (Suppl. 6A): 3–21, 1985
Bohnen JMA, Solomkin JS, Dellinger EP, Bjornson HS, Page CP. Guidelines for clinical care: anti—infective agents for intra—abdominal infection. Archives of Surgery 127: 83–89, 1992
Bootman JL, Zaske DE, Wertheimer AI, Rowland C. Cost of individualizing aminoglycoside dosage regimens. American Journal of Hospital Pharmacy 36: 368–370, 1979
Brook I. Enhancement of growth of aerobic and facultative bacteria in mixed infections with Bacteroides sp. Infection and Immunity 50: 929–931, 1985
Brook I, Hunter V, Walker RI. Synergistic effect of Bacteroides, Clostridium, Fusobacterium, anaerobic cocci, and aerobic bacteria on mortality and induction of subcutaneous abscess in mice. Journal of Infectious Diseases 149: 924–928, 1984
Broze B, De Mees J, Droissart R, Nolens VP, Staudt JP, et al. Randomized comparison of imipenem/cilastatin and ceftazidime in the empiric therapy of severe abdominal infections: a multicenter study. Acta Clinica Belgica 42: 431–436, 1987
Calandra GB, Wang C, Aziz M, Brown KR. The safety profile of imipenem/cilastatin: worldwide clinical experience ased on 3470 patients. Journal of Antimicrobial Chemotherapy 18 (Suppl.E): 193–202, 1986
Christen D, Bachmann P, Gerbulanos S. Imipenem/cilastatin versus amino—glycoside plus amoxicillin plus clindamycin in the treatment of serious post—operative infections. Scandinavian Journal of Infectious Diseases 52 (Suppl.): 11–14, 1987
Clissold SP, Todd PA, Campoli-Richards DM. Imipenem/cilastatin: a review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs 33: 183–241, 1987
Danziger LH, Creger RJ, Stellano TA, et al. A randomized trial of gentamicin and clindamycin vs imipenem—cilastatin in serious infections. Abstract. Drug Intelligence and Clinical Pharmacy 19: 455, 1985
Davey P, Hernanz C, Lynch W, Malek M, Byrne D. Human and non—financial costs of hospital—acquired infection. Journal of Hospital Infection 18 (Suppl. A): 79–84, 1991
Davey PG, Malek MM, Parker SE. Pharmacoeconomics of antibacterial treatment. PharmacoEconomics 1: 409–437, 1992
Destache CJ, Meyer SK, Bittner MJ, Hermann KG. Impact of a clinical pharmacokinetic service on patients treated with aminoglycosides: a cost—benefit analysis. Therapeutic Drug Monitoring 12: 419–426, 1990
Eisenberg JM, Koffer H, Glick HA, Connel ML, Loss LE, et al. What is the cost of nephrotoxicity associated with aminoglycosides? Annals ofInternal Medicine 107: 900–909, 1987
Erttmann M, Krausse R, Ullmann U. Pharmacokinetics of imipenem in patients undergoing major colon surgery. Infection 18: 367–371, 1990
Fry DE, Garrison RN, Heitsch RC, Calhoun K, Polk HC. Determinants of death in patients with intra—abdominal abscess. Surgery 88: 517–523, 1980
Gerecht WB, Henry NK, Muller SM, et al. Imipenem/cilastatin vs conventional therapy: a prospective controlled randomized study in non neutropenic bacteremic patients. Abstract. Presented at the 26th meeting of the Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, Oct. 1986
Geroulanos SJ, Stern A, Christen D, Buchmann P. Comparative study of imipenem/cilastatin vs. combination therapy in treating serious postoperative infections. Complications in Surgery 10: 37–39, 1991
Gill MA, Chenella FC, Heseltine PNR, Yellin AE, Berne TV, et al. Economic considerations in perforated and gangrenous appendicitis: imipenem—cilastatin versus clindamycin and gentamicin. Current Therapeutic Research 40: 393–402, 1986
Glazier HS, Creger RJ. A cost comparison of the treatment of surgical infections with imipenem/cilastatin versus the combination of clindamycin and gentamicin. Advances in Therapy 2: 73–81, 1985
Gorbach SL. Treatment ofintraabdominal sepsis. In Finegold SM (moderator). Management of anaerobic infections. Annals of Internal Medicine 83: 375–389, 1975
Gonzenbach HR, Simmen HP, Amgwerd R. Imipenem (N—F—thienamycin) versus netilimicin plus clindamycin. Annals of Surgery 205: 271–275, 1987
Guerra JG, Casalino E, Palomino JC, Barboza E, del Castillo M, et al. Imipenem/cilastatin vs. gentamicin/c1indamycin for the treatment of moderate to severe infections in hospitalized patients. Reviews of Infectious Diseases 7 (Suppl. 3): S463–S470, 1985
Guglielmo BJ, Brooks GF. Antmicrobial therapy: cost—benefit considerations. Drugs 38: 473–480, 1989
Hackford AW. Polymicrobial intraabdominal infection: medical/ surgical therapy. Infections in Surgery 9 (Suppl. 1): 40–45, 1990
Hackford AW, Tally FP, Reinhold RB, Barza M, Gorbach SL. Prospective study comparing imipenem—cilastatin with clindamycin and gentamicin for the treatment of serious surgical infections. Archives of Surgery 123: 322–326, 1988
Heseltine PNR, Yellin AE, Appleman MD, Gill MA, Chenella FC, et al. Imipenem therapy for perforated and gangrenous appendicitis. Surgery Gynecology and Obstetrics 162: 43–48, 1986
Jaresko GS, Barriere SL. Imipenem monotherapy versus combination therapy in the management of mixed bacterial infection: a critical appraisal. Pharmacotherapy 8: 324–333, 1988
Kager L, Nord CE. Imipenem/cilastatin in the treatment of intra abdominal infection: a review of world wide experience. Review of Infectious Diseases 7: (Suppl. 3): S518–S521, 1985
Klietmann W, Focht J, Nösner K. Retrospective resistance pattern of clinical isolates in vitro against imipenem and other antimicrobial agents between 1986 and 1989. Drug Investigation 3: 270–277, 1991
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical Care Medicine 13: 818–829, 1985
Leaper DJ. Treatment of acute bacterial peritonitis. Infections in Surgery 9 (Suppl. 1): 51–55, 1990
Leaper DJ, Kennedy RH, Sutton A, Johnson E, Roberts N. Treatment of acute bacterial peritonitis: a trial of imipenem/cilastatin against ampicillin—metronidazole—gentamicin. Scandinavian Journal of Infectious Diseases 52 (Suppl.): 7–10, 1987
Mandell LA, Turgeon P, Ronald A, et al. A randomized controlled trial of imipenem—cilastatin (I—C) vs clindamycin/tobramycin (CI/T) for intraabdominal and pelvic infections. Abstract. Presented at the 27th meeting of the Interscience Conference on Antimicrobial Agents and Chemotherapy, New York, Oct, 1987
Mathews A, Bailie GR. Clinical pharmacokinetics, toxicity and cost effectiveness analysis of aminoglycosides and aminoglycoside dosing services. Journal of Clinical Pharmacy and Therapeutics 12: 273–291, 1987
Meakins JL, Solomkin JS, Allo MD, Dellinger EP, Howard RJ, et al. A proposed classification of intra—abdominal infections. Stratification of etiology and risk for future therapeutic trials. Archives of Surgery 119: 1372–1378, 1984
Mouton Y, Deboscker Y, Bazin C, et al. Prospective randomized controlled study ofimipenem/cilastatin versus cefotaxime plus amikacin in lowere respiratory tract infections or bacteremia of intensive care patients. Abstract. Presented at the 27th meeting of the Interscience Conference on Antimicrobial Agents and Chemotherapy, New York, Oct. 1987
Nord CE. Incidence and significance of intraperitoneal aerobic and anaerobic bacteria. Clinical Therapeutics 12 (Suppl. B): 9–20, 1990
Nord CE, Kager L, Heimdahl A. Microbiological and clinical aspects on intra-abdominal infections. Scandinavian Journal of Gastroenterology 19 (Suppl.): 31, 1984
Norrby SR, Eriksson M, Ottosson E. Imipenem/cilastatin versus gentamicin/clindamycin: a cost effectiveness study. Scandinavian Journal of Infectious Diseases 18: 371–374, 1986
Norwegian Study Group. Imipenem/cilastatin as monotherapy in severe infections: comparison with cefotaxime in combination with metronidazole and cloxacillin. Scandinavian Journal of Infectious Diseases 19: 667–675, 1987
Pastel DA. Imipenem—cilastatin sodium, a broad—spectrum carbapenem antibiotic combination. Clinical Pharmacy 5: 719–736, 1986
Péze P, Mounier M, Cevallos R, Denis F. Êtude de la pénétration intrapéritonéale de l’imipénème/cilastatine au cours de péritonites aiguës avec perforation. Pathologie Biologie 38: 504–507, 1990
Poenaru D, De Santis M, Christou NV. Imipenem versus tobramycin — Antianaerobe antibiotic therapy in intra—abdominal infections. Canadian Journal of Surgery 33: 415–422, 1990
Potel G, Baron D. Imipenem/cilastatin for treatment of acute bacterial peritonitis: a French multicenter study. Complications in Surgery 10: 18–21, 1991
Satta G. Yalutazione dell’attività in vitro dell’Imipenem dopo circa 3 anni dall’inizio del suo impegiego nella pratica clinica. Scienzae Medicina 1 (Suppl.): 5–89, 1990
Saini S, Kellum F, O’Leary M, O’Donnell TF, Tally FP, et al. Improved localization and survival in patients with intraabdominal abscesses. American Journal of Surgery 145: 136–142, 1983
Scandinavian Study Group. Imipenem/cilastatin versus gentamicin/clindamycin for treatment of serious bacterial infections. Lancet 1: 868–871, 1984
Solomkin IS. Optimizing aminoglycoside therapy: is it worth the effort? Presented at a symposium on The Role of Monotherapy in Serious Surgical Infections, Scottsdale, Arizona, April 1, 1989
Solomkin JS, Christou NY, Dellinger EP, et al. A multicenter comparison of imipenem/cilastatin vs tobramycin/clindamycin for intra—abdominal sepsis. Abstract. Presented at the 27th meeting of the Interscience Conference on Antimicrobial Agents and Chemotherapy, New York, Oct. 1987
Solomkin IS, Dellinger EP, Christou NY, Busuttil RW. Results of a multicenter trial comparing imipenem/cilastatin to tobramycin/clindamycin for intra—abdominal infections. Annals of Surgery 212: 581–591, 1990
Solomkin JS, Fant WK, Rivera JO, Alexander JW. Randomized trial of imipenem/cilastatin versus gentamicin and clindamycin in mixed flora infections. American Journal of Medicine 78 (Suppl. 6A): 85–91, 1985
Solomkin IS, Meakins JL, Allo MD, Dellinger EP, Simmons RL. Antibiotic trials in intra—abdominal infections. A critical evaluation of study design and outcome reporting. Annals of Surgery 200: 29–39, 1984
Swedish Study Group. Immune response in patients with intraabdominal infections treated with imipenem. Infection 17: 369–373, 1989
Tally FP. Factors affecting the choice of antibiotics in mixed infections. Journal of Antimicrobial Chemotherapy 22 (Suppl. A): 87–100, 1988
Tally FP, Gorbach SL. Therapy of mixed anaerobic—aerobic infections: lessons from intra—abdominal sepsis. American Journal of Medicine 78 (Suppl. 6A): 145–153, 1985
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Benfield, P., Chrisp, P. Imipenem/Cilastatin. Pharmacoeconomics 1, 443–459 (1992). https://doi.org/10.2165/00019053-199201060-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-199201060-00005