Abstract
Ceftazidime-avibactam (Zavicefta®) is an intravenously administered combination of the third-generation cephalosporin ceftazidime and the novel, non-β-lactam β-lactamase inhibitor avibactam. In the EU, ceftazidime-avibactam is approved for the treatment of adults with complicated urinary tract infections (cUTIs) [including pyelonephritis], complicated intra-abdominal infections (cIAIs), hospital-acquired pneumonia (HAP) [including ventilator-associated pneumonia (VAP)], and other infections caused by aerobic Gram-negative organisms in patients with limited treatment options. This article discusses the in vitro activity and pharmacological properties of ceftazidime-avibactam, and reviews data on the agent’s clinical efficacy and tolerability relating to use in these indications, with a focus on the EU label. Ceftazidime-avibactam has excellent in vitro activity against many important Gram-negative pathogens, including many extended-spectrum β-lactamase-, AmpC-, Klebsiella pneumoniae carbapenemase- and OXA-48-producing Enterobacteriaceae and drug-resistant Pseudomonas aeruginosa isolates; it is not active against metallo-β-lactamase-producing strains. The clinical efficacy of ceftazidime-avibactam in the treatment of cUTI, cIAI and HAP (including VAP) in adults was demonstrated in pivotal phase III non-inferiority trials with carbapenem comparators. Ceftazidime-avibactam treatment was associated with high response rates at the test-of-cure visit in patients with infections caused by ceftazidime-susceptible and -nonsusceptible Gram-negative pathogens. Ceftazidime-avibactam was generally well tolerated, with a safety and tolerability profile consistent with that of ceftazidime alone and that was generally typical of the injectable cephalosporins. Thus, ceftazidime-avibactam represents a valuable new treatment option for these serious and difficult-to-treat infections.
Similar content being viewed by others
Administered intravenously at a fixed ceftazidime:avibactam ratio of 4:1 |
Has excellent in vitro activity against many important Gram-negative pathogens, including many ceftazidime-nonsusceptible Enterobacteriaceae and P. aeruginosa; not active against metallo-β-lactamase-producing strains or most Acinetobacter spp. isolates |
Non-inferior to carbapenem comparators in cUTI/acute pyelonephritis, cIAI (in combination with metronidazole) and HAP/VAP |
Effective against infections caused by ceftazidime-susceptible and -nonsusceptible pathogens |
Generally well tolerated, with a tolerability profile consistent with that of ceftazidime alone |
1 Introduction
The increasing prevalence of multidrug resistant (MDR) Gram-negative bacterial pathogens worldwide is a significant global public health concern [1,2,3]. Antimicrobial resistance among Gram-negative pathogens (in particular, resistance to β-lactam antimicrobials) is commonly driven by the production of β-lactamases, which can greatly limit treatment options for serious bacterial infections [4]. The increasing prevalence of extended-spectrum β-lactamase (ESBL)-producing pathogens has driven increased use of and reliance on carbapenems [3, 5]. The emergence and spread of carbapenemase-producing pathogens (including carbapenem-resistant Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacteriaceae) is thus of particular concern and has highlighted the urgent need for new antimicrobial agents [1, 6].
One strategy used to combat β-lactamase-producing pathogens has been to combine a β-lactam antimicrobial agent with a β-lactamase inhibitor [7]. However, classical β-lactamase inhibitors (i.e. clavulanic acid, tazobactam and sulbactam) lack activity against many important groups or classes of β-lactamases and, thus, first-generation β-lactam/β-lactamase inhibitor combinations are frequently ineffective against MDR pathogens [7]. Avibactam is a novel, non-β-lactam, β-lactamase inhibitor [8,9,10]. It has a broader spectrum of activity than classical β-lactamase inhibitors, with activity against Ambler class A, class C and some class D enzymes [8,9,10,11]. In vitro studies have shown that avibactam can restore the antimicrobial activity of the third-generation, extended-spectrum cephalosporin ceftazidime against many ESBL-, AmpC-, Klebsiella pneumoniae carbapenemase (KPC)- and OXA-48-producing Enterobacteriaceae and drug-resistant P. aeruginosa isolates [12,13,14,15,16,17].
Ceftazidime-avibactam (Zavicefta®) is an intravenously administered combination of ceftazidime and the β-lactamase inhibitor avibactam, administered at a fixed ceftazidime:avibactam ratio of 4:1 [18]. In the EU, ceftazidime-avibactam is approved for the treatment of adults with complicated urinary tract infection (cUTI), including pyelonephritis; complicated intra-abdominal infection (cIAI); hospital-acquired pneumonia (HAP), including ventilator-associated pneumonia (VAP); as well as for the treatment of other infections due to aerobic Gram-negative organisms in adult patients with limited treatment options [18]. Ceftazidime-avibactam is also approved in the USA (marketed as Avycaz®) in adults (aged ≥ 18 years) for the treatment of cIAI (in combination with metronidazole), cUTI (including pyelonephritis) or HAP/VAP caused by designated susceptible Gram-negative microorganisms [19].
This article reviews the therapeutic efficacy, safety and tolerability of ceftazidime-avibactam, with a focus on the EU label. The pharmacological properties of the agent are also summarized.
2 Antibacterial Activity
2.1 Mechanism of Action
Ceftazidime is an established, third-generation, broad-spectrum cephalosporin that, like other β-lactam antimicrobials, exerts its antibacterial effect by binding to penicillin-binding proteins (PBPs), thereby inhibiting peptidoglycan crosslinking during cell wall synthesis, leading to bacterial cell lysis and death [18, 20].
Avibactam is a first-in-class, non-β-lactam, β-lactamase inhibitor [8,9,10, 18]. It has no significant intrinsic antimicrobial activity itself [8]. Rather, it contributes to the activity of ceftazidime-avibactam by protecting ceftazidime from degradation by a variety of serine β-lactamases [8,9,10]. Avibactam acts through covalent acylation of its β-lactamase targets in a process that is slowly reversible, with deacylation (without hydrolysis) and release of intact avibactam [9, 21]. Avibactam has a broad spectrum of activity, inhibiting Ambler class A (e.g. TEM-1, CTX-M-15, KPC-2, KPC-3), class C (e.g. AmpC) and certain class D β-lactamases (e.g. OXA-10, OXA-48); it is not active against class B enzymes (metallo-β-lactamases) [8,9,10, 15, 18, 22,23,24].
2.2 In Vitro Activity
This section focuses on the in vitro antibacterial activity of ceftazidime-avibactam against clinically relevant isolates associated with approved indications for the drug, including cUTI, cIAI and HAP. Data are primarily drawn from the ongoing International Network For Optimal Resistance Monitoring (INFORM) global surveillance program, in which Gram-negative clinical isolates have been collected from sites across 40 countries (including 19 European countries) [16, 17, 25, 26]. Data presented in this section are for isolates collected between 2012 and 2014 inclusive. In INFORM, isolates were collected from patients with intra-abdominal, urinary tract, skin and soft-tissue, lower respiratory tract, and bloodstream infections. Isolates were tested for susceptibility to ceftazidime-avibactam (with avibactam at a fixed concentration of 4 µg/mL) and a range of comparative agents using broth microdilution panels following Clinical and Laboratory Standards Institute methodology. European Committee on Antimicrobial Susceptibility Testing (EUCAST) minimum inhibitory concentration (MIC) breakpoints for ceftazidime-avibactam for Enterobacteriaceae and P. aeruginosa are: susceptible, ≤ 8 µg/mL; resistant, > 8 µg/mL (with the avibactam concentration fixed at 4 µg/mL) [18].
The in vitro activity of ceftazidime-avibactam and a range of comparator agents against isolates of Enterobacteriaceae and P. aeruginosa collected in INFORM is summarized in Tables 1 and 2.
Ceftazidime-avibactam exhibits excellent in vitro activity against Enterobacteriaceae. Overall, 99.5% of isolates collected in INFORM in 2012–2014 were susceptible to ceftazidime-avibactam, which compared favourably with all of the other antimicrobials tested (Table 1) [16]. The activity of ceftazidime-avibactam was consistently high against all of the most commonly isolated individual pathogenic species or genera of Enterobacteriaceae, including Escherichia coli, K. pneumoniae, Enterobacter spp. and Proteus mirabilis. The MIC required to inhibit 90% of isolates (MIC90) for ceftazidime-avibactam against Enterobacteriaceae was 0.5 µg/mL, seven doubling dilutions lower than the MIC90 for ceftazidime alone (64 µg/mL). Among the 185 (0.5%) of 34,062 Enterobacteriaceae isolates tested that were nonsusceptible to ceftazidime-avibactam, 144 (77.8%) were metallo-β-lactamase positive. The vast majority of ceftazidime-avibactam nonsusceptible Enterobacteriaceae isolates (177/185; 95.7%) were also carbapenem nonsusceptible [16]. Furthermore, 83.5% of meropenem-nonsusceptible Enterobacteriaceae isolates were susceptible to ceftazidime-avibactam [25]. In contrast to ceftazidime and cefepime, ceftazidime-avibactam retained potent in vitro activity against Enterobacteriaceae isolates with ESBLs and/or plasmid-mediated AmpC β-lactamases (Table 1) [16]. Whereas the in vitro activities of carbapenems were substantially impacted by the presence of KPC enzymes, ceftazidime-avibactam retained excellent in vitro activity against KPC-positive Enterobacteriaceae, with 97.5% of such isolates susceptible to the drug combination (Table 1) [17]. The in vitro activity of ceftazidime-avibactam against metallo-β-lactamase-producing Enterobacteriaceae was very limited (MIC90, > 128 µg/mL) [25], which was as expected given that avibactam is not active against class B β-lactamases.
Ceftazidime-avibactam also exhibits excellent in vitro activity against P. aeruginosa. Overall, 92.0% of isolates collected in INFORM in 2012–2014 were susceptible to ceftazidime-avibactam, which compared favourably with all of the other β-lactam antimicrobials tested, including the carbapenems (Table 2) [26]. The MIC90 for ceftazidime-avibactam against P. aeruginosa isolates (8 µg/mL) was three doubling dilutions lower than that for ceftazidime alone (64 µg/mL). Ceftazidime-avibactam was active against 65 and 72% of ceftazidime-nonsusceptible and meropenem-nonsusceptible P. aeruginosa isolates in vitro [26]. Furthermore, 76% of KPC-producing P. aeruginosa isolates (n = 29) were susceptible to ceftazidime-avibactam [17].
In vitro data indicate that Acinetobacter spp. and Stenotrophomonas maltophilia are generally not susceptible to ceftazidime-avibactam [11, 18, 27]. In a study with European clinical isolates, ceftazidime-avibactam had an MIC90 against A. baumannii isolates (n = 30) of 64 µg/mL [27]. Ceftazidime (and thus ceftazidime-avibactam) has little or no in vitro activity against the majority of Gram-positive bacteria [18]. Similarly, the in vitro activity of ceftazidime and ceftazidime-avibactam against anaerobes is very limited [18].
In comparisons with the β-lactam/β-lactamase inhibitor combination ceftolozane-tazobactam, ceftazidime-avibactam appears to have lower MICs against ESBL-producing Enterobacteriaceae and higher MICs against P. aeruginosa isolates, although susceptibility rates were similar between the two agents [28, 29]. In contrast, ceftazidime-avibactam has better in vitro activity against carbapenem-resistant Enterobacteriaceae [29], with tazobactam lacking activity against AmpC β-lactamases, KPCs and OXA-carbapenemases [30].
2.3 In Vivo Activity
In vivo activity of ceftazidime-avibactam has been demonstrated in several animal studies, supporting the findings from in vitro studies. Human-simulated doses of ceftazidime-avibactam were shown to substantially decrease bacterial densities in models of thigh [31, 32] and lung [33] infections in immunocompetent [31, 32] and neutropenic [31,32,33] mice with infections caused by Enterobacteriaceae (including ESBL-, AmpC- and KPC-producing strains) [32] and P. aeruginosa [31, 33] clinical isolates. Ceftazidime-avibactam in vivo activity was also demonstrated in studies using a murine model of acute lethal septicaemia caused by isolates of Enterobacteriaceae producing ESBL [34], AmpC [34] or KPC [35] enzymes. In general, ceftazidime-avibactam in vivo activity in the murine models was pharmacodynamically predictable based on MIC values of the bacterial isolates [31,32,33].
2.4 Resistance
Based on limited available data, the potential for the selection of resistance to ceftazidime-avibactam appears to be relatively low [36, 37]. The most common mechanism of acquired resistance to ceftazidime-avibactam in clinically important Gram-negative pathogens is the production of β-lactamases that are refractory to inhibition by avibactam [e.g. class B enzymes (metallo-β-lactamases) and many class D enzymes] [16, 26]. Ceftazidime-avibactam resistance has also been observed in strains with mutations in AmpC or carbapenemase enzymes [36,37,38,39,40]. Some cases of ceftazidime-avibactam resistance have been linked to mutations in plasmid-borne KPC-3 [39, 40]. Interestingly, it was found that some KPC-3 mutations that conferred ceftazidime-avibactam resistance were associated with decreases in the MICs for carbapenems and other β-lactam antibiotics [39, 40].
In INFORM, 0.5% of Enterobacteriaceae isolates (Table 1) and 8% of P. aeruginosa isolates (Table 2) collected in 2012-2014 were nonsusceptible to ceftazidime-avibactam. Forty-one of the 185 ceftazidime-avibactam-nonsusceptible Enterobacteriaceae isolates did not contain a metallo-β-lactamase [16], and no acquired β-lactamase gene was identified in 199 of the 563 ceftazidime-avibactam-nonsusceptible P. aeruginosa isolates [26], suggesting that resistance to ceftazidime-avibactam may be conferred via other mechanisms. Besides those mediated through β-lactamases, other potential resistance mechanisms include changes in the drug target (e.g. mutant or acquired PBPs), decreased outer membrane permeability of either component of the drug combination, or active efflux of either component [18, 41,42,43].
2.5 Pharmacokinetic/Pharmacodynamic Considerations
Consistent with other β-lactam antimicrobials, the best predictor of antimicrobial activity of ceftazidime is the percentage of the dosing interval that the free-drug concentration remains above the ceftazidime-avibactam MIC (%fT > MIC) [18]. The pharmacokinetic-pharmacodynamic (PK-PD) index for avibactam is the percentage of the dosing interval that the free-drug concentration remains above a certain threshold concentration (%fT > CT) [18]. Analysis using a population pharmacokinetic model found that the ceftazidime %fT > MIC required to likely lead to a favourable outcome in patients with HAP was > 45% [44]. Furthermore, a study using murine neutropenic thigh and lung infection models with ceftazidime-resistant P. aeruginosa found the best approximation of the threshold concentration of avibactam was 1 µg/mL [45]. In experiments in the murine lung infection model, a static effect in vivo was achieved with a %fT > CT 1 µg/mL of approximately 20%, with approximately 24 and 30% required for 1- and 2-log kills, respectively [45].
Using a joint PK-PD target of ceftazidime 50%fT > MIC at a ceftazidime-avibactam MIC of 8 µg/mL and avibactam 50%fT > CT at 1 µg/mL, a probability of PK-PD target attainment (PTA) simulation using population pharmacokinetic models found that high (> 95%) PTA was predicted for cUTI, cIAI or HAP patients with normal renal function using a dosage regimen of ceftazidime-avibactam 2.5 g administered in a 2-h intravenous infusion every 8 h [i.e. the regimen used in pivotal phase III trials (see Sect. 4) and the EMA-approved dosage for patients with creatinine clearance (CLCR) ≥ 51 mL/min (see Sect. 6)] [46].
3 Pharmacokinetic Properties
The pharmacokinetics of ceftazidime and avibactam are not affected when the drugs are administered in combination [18, 47, 48]. Both drugs exhibit approximate dose linearity when administered at clinically relevant doses [18, 47, 49].
Following a single intravenous infusion of ceftazidime-avibactam 2.5 g over 2 h to healthy adult male subjects, maximum plasma concentrations (Cmax) of ceftazidime and avibactam were 88.1 and 15.2 µg/mL, with Cmax for both drugs reached near the end of the 2-h infusion [48]. Area under the plasma concentration-time curve (AUC) values for ceftazidime and avibactam were 289.0 and 42.1 µg·h/mL. There was no appreciable accumulation of either drug following multiple intravenous infusions of ceftazidime-avibactam 2.5 g every 8 h over 11 days [48]. Furthermore, no time-dependent pharmacokinetics were apparent. Both drugs reached steady-state pharmacokinetics prior to day 4 [48]. Following multiple infusions of ceftazidime-avibactam 2.5 g every 8 h (in 30-min infusions) in healthy male subjects, trough concentrations of ceftazidime and avibactam at day 10 were 4.5 and 0.25 µg/mL, respectively [47].
Both ceftazidime and avibactam exhibit low levels of human protein binding (≈ 10 and 8%, respectively) [18]. At steady state, volumes of distribution of ceftazidime and avibactam were approximately 22 and 18 L, respectively [18]. A phase I study in healthy adult males showed that both drugs penetrate dose-proportionally into bronchial epithelial lining fluid, each reaching concentrations ≈ 30% (or higher at lower plasma concentrations [50]) of those observed in plasma [49].
Mean terminal elimination half-lives (t½) of ceftazidime and avibactam were 3.5 and 2.3 h after a single dose and 2.8 and 2.8 h after multiple dosing of ceftazidime-avibactam 2.5 g every 8 h in healthy adult male subjects [48]. Neither ceftazidime or avibactam appear to be metabolized to any great extent, with both drugs primarily eliminated unchanged in the urine [11, 18, 51]. Ceftazidime excretion occurs via glomerular filtration [18]; for avibactam, the renal clearance rate (158 mL/min) is greater than the glomeration filtration rate for unbound drug (109.5 mL/min), indicating that clearance involves active tubular secretion in addition to glomerular filtration [51]. Ceftazidime and avibactam are both removed by haemodialysis [18].
As expected given the renal elimination of ceftazidime and avibactam, the pharmacokinetics of both drugs are impacted by renal impairment. For both ceftazidime [52] and avibactam [53], an increasing degree of renal impairment was associated with decreasing clearance and increasing drug exposure and t½. Ceftazidime-avibactam dosage reductions are required in patients with estimated CLCR ≤ 50 mL/min (see Sect. 6) [18].
Ceftazidime pharmacokinetics are not affected by mild to moderate hepatic impairment in the absence of renal impairment [18]. Avibactam pharmacokinetics have not been studied in patients with hepatic impairment. However, given that neither ceftazidime or avibactam appear to undergo significant hepatic metabolism, hepatic impairment is not expected to have any clinically relevant effect on the systemic clearance of either drug, and no ceftazidime-avibactam dosage adjustment is recommended based on this factor [18].
The pharmacokinetics of ceftazidime-avibactam are not affected to a clinically relevant extent by gender, age, bodyweight or race, and no dosage adjustment is required based on these factors [18].
Given the apparent lack of significant hepatic metabolism of the component drugs, ceftazidime-avibactam is considered to have low potential for drug-drug interactions [11, 18, 48, 51]. No interaction between ceftazidime-avibactam and metronidazole was observed in a phase I clinical trial [48]. Avibactam is a substrate of organic anion transporters 1 and 3 (OAT1 and OAT3) in vitro, and avibactam uptake is inhibited by probenecid, a potent OAT1/OAT3 inhibitor [18, 51]. In the absence of a clinical interaction study, avibactam and probenecid co-administration is not recommended [18, 51].
4 Therapeutic Efficacy
Ceftazidime-avibactam has been evaluated in several randomized, controlled phase III trials. In all trials discussed in this section, across all indications, ceftazidime-avibactam was administered at a dosage of 2.5 g (i.e. ceftazidime 2 g and avibactam 0.5 g) every 8 h as a 2-h intravenous infusion (with dosage reductions applied for renal impairment) [55,56,57,58,59]. Most of these trials had separate primary endpoints based on EMA and US FDA guidance [55, 57]. Given the scope of this article, discussion on primary endpoint analyses in this section focuses on the EMA-based endpoints. Findings from data on FDA-based primary endpoints (where applicable) are provided in tables for completeness. Since MIC breakpoints for ceftazidime-avibactam had not been formally established when the phase III trials began, pathogen susceptibility to ceftazidime-avibactam was determined based on then provisional breakpoints (MIC ≤ 8 µg/mL, susceptible; MIC > 8 µg/mL, resistant). These breakpoints match those now formally established by EUCAST (see Sect. 2.2).
4.1 Complicated Urinary Tract Infections
The efficacy of ceftazidime-avibactam in the treatment of adults with cUTI (including pyelonephritis) was evaluated in the RECAPTURE-1 and RECAPTURE-2 trials (Sect. 4.1.1), two randomized, double-blind, double-dummy, multinational, phase III trials of identical design that compared ceftazidime-avibactam with doripenem [55]. Data from these trials were combined into a single dataset for evaluation of the non-inferiority of ceftazidime-avibactam versus doripenem. Evidence for the efficacy of ceftazidime-avibactam is also available from the REPRISE trial (Sect. 4.1.2), a randomized, open-label phase III trial that evaluated the efficacy of ceftazidime-avibactam versus best available therapy in patients with cUTI (92% of patients) or cIAI (8% of patients) caused by ceftazidime-nonsusceptible Enterobacteriaceae or P. aeruginosa [56].
4.1.1 RECAPTURE-1 and RECAPTURE-2 Trials
Eligible participants were adults (aged 18–90 years) with cUTI (see Table 3 for definition) or acute pyelonephritis requiring hospitalization for treatment with intravenous antibiotic therapy. Diagnosis was based on the presence of pyuria and a positive urine culture [1–2 Gram-negative uropathogens at ≥ 105 colony-forming units (CFU)/mL]. Patients could be enrolled before urine culture results became available, provided that results were expected to be positive (based on urinalysis and clinical findings), the study treatments were considered appropriate empiric therapy, and a urine Gram stain showed the presence of Gram-negative bacilli and no Gram-positive organisms [55]. Patients were excluded if urine culture results were available showing one or more Gram-negative uropathogen(s) resistant to either study drug, a Gram-positive organism present at ≥ 105 CFU/mL or a confirmed fungal UTI (> 103 CFU/mL). Other key exclusion criteria included a urinary catheter or device that would not be removed during the study treatment period, complete obstruction of any portion of the urinary tract, perinephric or intrarenal abscess, prostatitis, urinary diversion or vesicoureteral reflux, violation of antibiotic restriction criteria, CLCR ≤ 30 mL/min or evidence of abnormal liver function [55].
In the trials, patients (n = 1033 in total) were randomized 1:1 to receive ceftazidime-avibactam 2.5 g every 8 h as a 2-h intravenous infusion or doripenem 500 mg every 8 h as a 1-h intravenous infusion (followed by a 1-h placebo infusion to maintain blinding) [55]. Dosages of ceftazidime-avibactam and doripenem were both reduced in patients with moderate renal impairment (CLCR > 30 to ≤ 50 mL/min). After ≥ 5 days of intravenous study drug therapy, patients meeting prespecified criteria for clinical improvement could be switched to appropriate oral antibiotic therapy. Study treatment (intravenous therapy plus optional oral therapy) was administered for up to 10 days, or up to 14 days for patients with bacteraemia at baseline [55].
The primary endpoint under EMA guidance was the proportion of patients with a favourable per-patient microbiological response (i.e. eradication, defined as a urine culture showing < 104 CFU/mL of the original uropathogen, and resolution of bacteraemia if present) at the test-of-cure (TOC) visit (21–25 days post-randomization) in the microbiological modified intent-to-treat (mMITT) population (Table 3) [55]. Separate co-primary endpoints were defined under FDA guidance (see Table 3).
Patient demographics and baseline disease characteristics were well balanced between treatment groups [55]. The majority (75%) of randomized patients were from Eastern Europe, 5% were from North America and Western Europe and 20% were from the rest of the world. The mMITT population (n = 810; 393 and 417 patients in the ceftazidime-avibactam and doripenem groups, respectively) comprised 78% of randomized patients. Twenty-eight percent of patients in the mMITT population had a diagnosis of cUTI without pyelonephritis and 72% had acute pyelonephritis [64 patients (7.9%) had acute pyelonephritis and also met symptom criteria for cUTI]. At baseline, 8.8% of patients in the mMITT population had bacteraemia [55].
The vast majority of patients in the mMITT population (801/810) had a single baseline uropathogen, with the pathogens typical of those associated with cUTI [55]. More than 95% of patients had an Enterobacteriaceae isolated from urine, most commonly E. coli (73.8%). E. coli was also the most common pathogen isolated from blood. Other common Gram-negative uropathogens were K. pneumoniae (12.3% of patients in the mMITT population), P. aeruginosa (4.7%), P. mirabilis (3.7%) and Enterobacter cloacae (3.0%). At baseline, 19.1% of patients had ESBL-positive Enterobacteriaceae uropathogens. Ceftazidime-nonsusceptible pathogens (predominantly E. coli or K. pneumoniae) were isolated from 159 (19.6%) patients at baseline, and pathogens that were nonsusceptible to ceftazidime-avibactam or doripenem were isolated from 27 (3.3%) patients (1 isolate nonsusceptible to ceftazidime-avibactam, 19 to doripenem, 5 to both study drugs, and 7 with missing data) [55].
Ceftazidime-avibactam was non-inferior to doripenem for the treatment of hospitalized adults with cUTI or acute pyelonephritis, based on both the EMA-defined primary endpoint (with a prespecified non-inferiority margin of − 12.5%) and the US FDA-defined co-primary endpoints (with prespecified non-inferiority margins of − 10.0%) (Table 3) [55]. In addition, ceftazidime-avibactam was found to be superior to doripenem (at the 5% significance level) for the EMA-defined primary endpoint, given that the lower limit of the 95% confidence interval for the treatment difference was greater than zero (Table 3). The per-patient microbiological eradication rate was also higher for the ceftazidime-avibactam group versus the doripenem group at the late follow-up (LFU; 45–52 days post-randomization) visit [68.2 vs. 60.9%; between-group difference (BGD), 7.3%; 95% CI 0.68–13.81] [55]. In an exploratory endpoint analysis using a more stringent cutoff for eradication (i.e. < 103 CFU/mL) under EMA guidance, a favourable microbiological response at the TOC visit was achieved in 76.1% of ceftazidime-avibactam recipients and 69.8% of doripenem recipients (mMITT population), again with the lower limit of the 95% confidence interval for the treatment difference greater than zero (BGD 6.3%; 95% CI 0.17–12.38) [55].
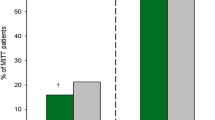
Per-pathogen eradication rates at the TOC visit for ceftazidime-nonsusceptible and -susceptible pathogens and the most common individual pathogens are shown in Fig. 1. Among patients with ceftazidime-nonsusceptible pathogens, per-patient eradication rates for ceftazidime-avibactam versus doripenem were 62.7 vs. 60.7% at the TOC visit (BGD 2.0%; 95% CI − 13.18 to 16.89) and 61.3 vs. 45.2% at the LFU visit (BGD 16.1%; 95% CI 0.50–30.89) [55]. Eradication rates for the ceftazidime-avibactam versus doripenem groups among patients with ceftazidime-susceptible pathogens were 81.0 vs. 73.0% at the TOC visit (BGD 8.0%; 95% CI 1.50–14.48) and 69.9 vs. 64.1% at the LFU visit (BGD 5.8%; 95% CI − 1.46 to 13.05) [55]. Clinical cure was sustained at the LFU visit in 93.0 and 91.5% of ceftazidime-avibactam and doripenem recipients who were cured at the TOC visit [55].
Per-pathogen favourable microbiological response (eradication) rates at the test-of-cure visit in RECAPTURE trials in patients with cUTI (mITT population) [55]. Individual pathogens shown are those identified in ≥ 10 patients. Total patient numbers in each treatment arm are shown above the bars. BL baseline, CAZ-NS ceftazidime-nonsusceptible, CAZ-S ceftazidime-susceptible
4.1.2 REPRISE Trial
REPRISE was a pathogen-directed study that specifically focused on the efficacy of ceftazidime-avibactam in the treatment of infections caused by ceftazidime-nonsusceptible pathogens [56]. Eligible participants were adults (aged 18–90 years) with cUTI (including pyelonephritis) or cIAI caused by a ceftazidime-nonsusceptible Gram-negative pathogen that had been isolated from an appropriate culture within 5 days prior to screening. Key exclusion criteria included an estimated CLCR < 6 mL/min, evidence of abnormal liver function, or an infection caused by a Gram-negative pathogen that was unlikely to respond to ceftazidime-avibactam (e.g. Acinetobacter or Stenotrophomonas species). In total, 333 patients (306 with cUTI, 27 with cIAI) were randomized (1:1) to receive ceftazidime-avibactam 2.5 g (every 8 h in a 2-h intravenous infusion) for 5–21 days or best available therapy (as determined by the investigator, based on standard of care and local label recommendations). Patients in the ceftazidime-avibactam group with cIAI also received metronidazole 500 mg (as a 1-h intravenous infusion every 8 h) for coverage of anaerobic pathogens. Ceftazidime-avibactam dosage reductions were applied for patients with estimated CLCR 6–50 mL/min. The vast majority (97%) of patients in the best available therapy group received a carbapenem antibiotic (96% as monotherapy), most commonly imipenem (50 and 33% of patients with cUTI and cIAI) or meropenem (37 and 60%) [56].
The primary endpoint of the trial was the proportion of patients achieving a clinical cure (defined as complete resolution or substantial improvement of signs and symptoms of the index infection, with no need for further antibacterial therapy) at the TOC visit (7–10 days after the last infusion of study therapy) in the mMITT population (i.e. all patients with cUTI or cIAI with ≥ 1 confirmed ceftazidime-nonsusceptible Gram-negative pathogen, and who received ≥ 1 dose of study drug) [56]. Given the difficulty in recruiting a sufficiently large number of patients with infections caused by ceftazidime-nonsusceptible Gram-negative pathogens, no formal statistical comparisons were planned or performed. Rather, the study was used to generate descriptive estimates of ceftazidime-avibactam efficacy based on comparisons using confidence intervals for the efficacy of best available therapy [56].
Among randomized patients, 80% were from Eastern Europe, 5% were from North America and Western Europe and 15% were from the rest of the world [56]. The mMITT population consisted of 302 patients (281 with cUTI, 21 with cIAI), with patient demographics and baseline disease characteristics well balanced between the two treatment groups. Besides the primary endpoint analysis, discussion in this section focuses on patients with cUTI since the small number of patients in the study with cIAI limited interpretation of results for the cIAI subpopulation. Among patients with cUTI, 96% of infections were monomicrobial, and 48% of patients had had previous antibiotic therapy [56]. The majority of patients (94%) were infected with Enterobacteriaceae, with K. pneumoniae (43% of patients) and E. coli (41%) the most commonly isolated pathogens. Seven percent of patients were infected with P. aeruginosa at baseline. Among all Enterobacteriaceae pathogens isolated from patients with cUTI (including study-qualifying ceftazidime-nonsusceptible pathogens and any other pathogens), 264 (97.4%) of 271 isolates were nonsusceptible to ceftazidime (MIC ≥ 8 µg/mL). In comparison, four (1.5%) of 266 Enterobacteriaceae tested were nonsusceptible to ceftazidime-avibactam (MIC > 8 µg/mL) [56].
In the primary endpoint analysis, a similar proportion of patients in the ceftazidime-avibactam and best available therapy groups achieved a clinical cure at the TOC visit [90.9% (95% CI 85.6–94.7) vs. 91.2% (95% CI 85.9–95.0)] [56]. In the subgroup of patients with cUTI, again the proportion of patients achieving a clinical cure at the TOC visit was similar in the ceftazidime-avibactam and best available therapy groups [91.7% (95% CI 86.3–95.4) vs. 94.2% (95% CI 89.3–97.2)]. The proportions of patients who were clinically cured fell somewhat over subsequent follow-up visits (secondary endpoints), but remained similar between the ceftazidime-avibactam and best available therapy treatment groups [88.2 vs. 88.3% at follow-up visit 1 (21–25 days post randomization); 85.4 vs. 86.1% at follow-up visit 2 (28–32 days post randomization)]. When analysed by baseline pathogen (secondary endpoint), clinical cure rates at the TOC visit in the ceftazidime-avibactam and best available therapy groups were: K. pneumoniae, 54/55 (98%) vs. 61/65 (94%); E. coli, 53/59 (90%) vs. 54/57 (95%); P. aeruginosa, 12/14 (86%) vs. 5/5 (100%); other Gram-negative pathogens, 17/20 (85%) vs. 15/16 (94%) [56].
Another key secondary endpoint of the trial was the proportion of patients with a favourable per-patient microbiological response [defined as eradication (i.e. absence, or urine quantification < 104 CFU/mL) of the causative pathogen from the infection site] at the TOC visit. Among patients with cUTI in the mMITT population, a numerically higher proportion of patients in the ceftazidime-avibactam group (81.9%; 95% CI 75.1–87.6) than in the best available therapy group (64.2%; 95% CI 56.0–71.9) achieved a favourable microbiological response at the TOC visit [56].
4.2 Complicated Intra-Abdominal Infections
Evidence for the efficacy of ceftazidime-avibactam in the treatment of cIAI in adults is primarily drawn from the RECLAIM-1 and RECLAIM-2 trials, two randomized, double-blind, double-dummy, multinational, phase III trials of identical design which investigated the non-inferiority of ceftazidime-avibactam plus metronidazole versus meropenem (Sect. 4.2.1) [57]. Data from the RECLAIM-1 and -2 trials were combined into a single inferential dataset. Further evidence is available from the phase III RECLAIM-3 trial that investigated the non-inferiority of ceftazidime-avibactam plus metronidazole versus meropenem in adult patients in Asian populations (Sect. 4.2.2) [58].
4.2.1 RECLAIM-1 and RECLAIM-2 Trials
Hospitalized adults (aged 18–90 years) with a cIAI diagnosis were eligible for enrolment. Patients could be enrolled intra- or post-operatively with visual confirmation of an intra-abdominal infection associated with peritonitis or pre-operatively with confirmation of infection by surgical intervention within 24 h of study entry. Patients with a CLCR ≤ 30 mL/min or other potentially important laboratory findings were excluded, as were patients with an Acute Physiology and Chronic Health Evaluation (APACHE) II score > 30. Other key exclusion criteria included systemic antibacterial therapy during the 72-h period prior to study entry unless patients had a new infection or had failed the previous treatment regimen [57].
In the trials, patients (n = 1066 in total) were randomized 1:1 to receive ceftazidime-avibactam 2.5 g every 8 h as a 2-h intravenous infusion followed by metronidazole 500 mg (for coverage of anaerobic pathogens) as a 1-h intravenous infusion every 8 h, or meropenem 1000 mg as a 30-min intravenous infusion every 8 h (with placebo infusions as appropriate to maintain blinding) [57]. Dosages of ceftazidime-avibactam and meropenem were reduced in patients with moderate renal impairment (CLCR > 30 to ≤ 50 mL/min) at baseline [8.1% of patients in the modified intent-to-treat (MITT) population (Table 4)]. Intravenous study treatment was administered for 5–14 days. In addition, open-label vancomycin, linezolid, or daptomycin could be added to either regimen (based on the investigator’s discretion) if Enterococcus spp. or methicillin-resistant Staphylococcus aureus was isolated or suspected. Randomization was stratified based on baseline APACHE II score (≤ 10 or > 10 to ≤ 30) and by geographical region (North America and Western Europe, Eastern Europe, or rest of the world) [57].
The primary endpoint was the clinical cure rate at the TOC visit (28–35 days after randomization) assessed (under EMA guidance) in the MITT and clinically evaluable at the time of the TOC visit (CE) populations (Table 4) [57]. In the primary efficacy analysis, the non-inferiority of ceftazidime-avibactam plus metronidazole versus meropenem was assessed with a prespecified (EMA-suggested) non-inferiority margin of − 12.5%.
There were no clinically significant differences in demographics or baseline disease characteristics between treatment groups in any of the primary analysis sets [11, 57]. The majority of patients (60%) in the MITT population were from Eastern Europe, 16% were from North America and Western Europe and 24% were from the rest of the world [57]. Most patients (84%) had an APACHE II score of ≤ 10. The most common primary diagnoses in the MITT population were appendiceal perforation (41%), acute gastric and duodenal perforation (19%) and cholecystitis (16%). Approximately half of the patients were enrolled preoperatively [11]. Sixty-two percent of patients had received prior antibacterial therapy in the 72 h before randomization, the vast majority (91%) of these for a duration of ≤ 24 h [57]. In the MITT population, 40% of infections were monomicrobial and 40% were polymicrobial, with no qualifying pathogen identified in 20% of patients. At baseline, 4.2% of patients in the ceftazidime-avibactam plus metronidazole group and 2.7% of patients in the meropenem group had bacteraemia [57].
The mMITT population represented 77% of randomized patients [57]. Baseline pathogens isolated were typical of those associated with cIAI. One or more Enterobacteriaceae pathogens were isolated from the blood or intra-abdominal site in 83% of patients in the mMITT population, most commonly E. coli (67.6% of patients) and K. pneumoniae (12.2%). P. aeruginosa was isolated from 8.6% of mMITT patients [57].
Ceftazidime-nonsusceptible aerobic Gram-negative pathogens were isolated from 111 patients (13.5%) in the mMITT population [57]. Almost all of these isolates had a ceftazidime-avibactam MIC of ≤ 8 µg/mL. The ceftazidime-avibactam MIC90 among ceftazidime-nonsusceptible Enterobacteriaceae was 2 µg/mL. Among patients with ceftazidime- nonsusceptible pathogens, ≈ 80 and ≈ 3% had ESBL-positive and metallo-β-lactamase-positive infections, respectively [57]. Of all Gram-negative baseline pathogens, nine isolates (four Enterobacteriaceae, one Comamonas testosteroni and four P. aeruginosa) were nonsusceptible to ceftazidime-avibactam and eight isolates (five Enterobacteriaceae, one Burkholderia cepacia and two P. aeruginosa) were nonsusceptible to meropenem [57]. Anaerobic pathogens were isolated from 32% of patients mMITT population, and Gram-positive aerobes were isolated from 38% of patients. The mean duration of study drug treatment was 8.0 and 8.3 days in the ceftazidime-avibactam plus metronidazole and meropenem groups, respectively [57].
Ceftazidime-avibactam plus metronidazole was non-inferior to meropenem in the treatment of hospitalized adults with cIAI based on the clinical cure rate at the TOC visit (primary endpoint), with the lower limit of the 95% confidence interval for the between-group difference meeting the prespecified non-inferiority margin [i.e. above − 12.5%] (Table 4) [57]. Clinical cure rates for ceftazidime-avibactam plus metronidazole versus meropenem-treated patients were also analysed at the end-of-treatment (up to 24 h after the last infusion) and LFU (42–49 days after randomization) visits (secondary endpoints). Although these secondary endpoints were not formally assessed against a non-inferiority margin, the lower limits of the 95% confidence intervals for the between-group differences were above − 10% in both analysis sets, supporting the findings from the primary endpoint analysis [57].
Clinical cure rates at the TOC visit were generally similar between the ceftazidime-avibactam plus metronidazole and meropenem groups when analysed by baseline pathogen in the mMITT population: Enterobacteriaceae, 81.4 vs. 86.4% (including E. coli, 80.4 vs. 87.0%, and K. pneumoniae, 78.4 vs. 75.5%); P. aeruginosa, 85.7 vs. 94.4%, anaerobes, 79.9 vs. 81.0%, Gram-positive aerobes, 80.3 vs. 79.5% [57].
Ceftazidime-avibactam plus metronidazole also demonstrated efficacy in the treatment of cIAI involving ceftazidime-nonsusceptible Gram-negative pathogens [57]. Patients in ceftazidime-avibactam plus metronidazole group with infections involving ceftazidime-nonsusceptible and ceftazidime-susceptible pathogens had clinical cure rates of 83.0% and 82.0%, respectively, compared with rates of 85.9 and 87.7%, respectively, in the meropenem group [57].
4.2.2 RECLAIM-3 Trial
RECLAIM-3 was a randomized, double-blind, double-dummy phase III study of similar design to the RECLAIM-1 and -2 trials [58]. It included 441 adult patients (aged 18–90 years) hospitalized with cIAI in China (61% of patients), the Republic of Korea (24%) and Vietnam (15%), with participants randomized (1:1) to ceftazidime-avibactam plus metronidazole or meropenem according to the same regimens as in RECLAIM-1 and -2 (see Sect. 4.2.1). The primary endpoint of RECLAIM-3 was the clinical cure rate at the TOC visit (28–35 days after randomization) in the CE population (with assessment of the non-inferiority of ceftazidime-avibactam plus metronidazole versus meropenem with a prespecified non-inferiority margin of − 12.5%) [58].
Patient demographics and baseline disease characteristics were generally well balanced between treatment groups [58]. In the ceftazidime-avibactam plus metronidazole and meropenem groups in the MITT population, 39 and 47% of patients had monomicrobial infections, 27 and 24% had polymicrobial infections, and 34 and 29% had no study-qualifying pathogen identified. One or more Enterobacteriaceae were isolated from the blood and/or intra-abdominal site in 81.0% of patients in the mMITT population (n = 295), most commonly E. coli (59% of patients) and K. pneumoniae (21%). P. aeruginosa was isolated from 13% of patients in the mMITT population. Twenty percent of patients in the mMITT population had an infection involving a ceftazidime-nonsusceptible aerobic Gram-negative pathogen [58]. The mean duration of study drug treatment was 6.9 and 7.3 days in the ceftazidime-avibactam plus metronidazole and meropenem groups, respectively [58].
Non-inferiority of ceftazidime-avibactam plus metronidazole to meropenem was demonstrated in the primary endpoint analysis [58]. The clinical cure rate at the TOC visit in the CE population was 93.8% (166/177 patients) in the ceftazidime-avibactam plus metronidazole group and 94.0% (173/184) in the meropenem group (BGD − 0.2%; 95% CI − 5.53 to 4.97), with the lower limit of the between-group difference 95% confidence interval meeting the prespecified non-inferiority margin (i.e. above − 12.5%). Although not formally assessed against a non-inferiority margin, secondary endpoint analyses evaluating clinical cure rates between the ceftazidime-avibactam plus metronidazole and meropenem groups in the CE population at the end-of-treatment (within 24 h of the last study drug infusion) and at LFU (42–49 days after randomization) visits were consistent with the primary endpoint analysis [58].
In another secondary endpoint analysis, it was shown that the clinical cure rate at the TOC visit in patients in the ceftazidime-avibactam plus metronidazole group was similar between patients with ceftazidime-susceptible [70/76 (92.1%)] and ceftazidime-nonsusceptible [22/23 (95.7%)] Gram-negative pathogens [extended microbiologically evaluable population (i.e. patients in the CE population with ≥ 1 Gram-negative baseline pathogen regardless of susceptibility)] [58].
4.3 Hospital-Acquired Pneumonia
The efficacy of ceftazidime-avibactam in the treatment of adults with hospital-acquired pneumonia (HAP), including ventilator-associated pneumonia (VAP), was evaluated in the randomized, double-blind, double-dummy, multinational, phase III REPROVE trial, which investigated the non-inferiority of ceftazidime-avibactam versus meropenem [59]. In the trial, 879 adult patients (aged 18–90 years) with HAP (including VAP) were randomized 1:1 to receive ceftazidime-avibactam 2.5 g every 8 h as a 2-h intravenous infusion or meropenem 1000 mg every 8 h as a 30-min intravenous infusion for 7–14 days. Doses were reduced for renal function impairment. Randomization was stratified based on infection type (VAP or non-VAP HAP) and geographical region (Western Europe, Eastern Europe, China, or rest of the world) [59].
The primary endpoint of the trial under EMA guidance was the clinical cure rate at the TOC visit (21–25 days post-randomization) assessed in two co-primary analysis populations, the clinically modified intent-to-treat (cMITT) population (excluding patients with only non-target pathogens) and the CE subset of the cMITT population (i.e. those with an adequate course of treatment and no significant protocol deviations) [59]. In the primary efficacy analysis, the non-inferiority of ceftazidime-avibactam versus meropenem was assessed with a prespecified (EMA-suggested) non-inferiority margin of − 12.5%.
Baseline characteristics were well balanced between the treatment groups [59]. Most patients (86%) had an APACHE II score of < 20, and 34% of patients had VAP. The most common Gram negative pathogens isolated at baseline in the mMITT population were K. pneumoniae (37%) and P. aeruginosa (30%). Twenty-eight percent of patients had one or more isolate that was nonsusceptible to ceftazidime [59].
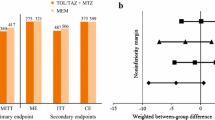
Ceftazidime-avibactam was non-inferior to meropenem in the treatment of adult patients with HAP (including VAP) based on the clinical cure rate at the TOC visit in both the cMITT (p = 0.007) and CE (p < 0.001) populations (Fig. 2a) [59]. Clinical cure at the TOC visit was experienced by 68.8 vs. 73.0% of ceftazidime-avibactam and meropenem recipients in the cMITT population (BGD − 4.2%; 95% CI − 10.76 to 2.46), and by 77.4 vs. 78.1% of ceftazidime-avibactam and meropenem recipients in the CE population (BGD − 0.7%; 95% CI − 7.86 to 6.39), with ceftazidime-avibactam meeting the prespecified non-inferiority margin of − 12.5% for both analysis populations. Subgroup analyses in non-VAP and VAP patients appeared to be consistent with the primary endpoint analyses (Fig. 2a). Clinical cure rates were also similar between ceftazidime-avibactam- and meropenem-treated patients across other prespecified subgroups, including those based on geographical region, APACHE II score (10–19 vs. 20–30) or previous systemic antibiotic use [59].
Clinical cure rates at test-of-cure visit in the phase III REPROVE trial in patients with hospital-acquired pneumonia (including VAP) [59]. a Primary efficacy and subgroup analyses and b by susceptibility of baseline isolates. Values above columns indicate patient numbers. †Non-inferior to meropenem. CAZ-NS ceftazidime-nonsusceptible, CAZ-S ceftazidime-susceptible, CE clinically evaluable population, cMITT clinically modified intent-to-treat population, mMITT microbiological modified intent-to-treat population, TOC test-of-cure, VAP ventilator-associated pneumonia
Per-pathogen clinical cure rates at TOC were similar between ceftazidime-avibactam recipients with ceftazidime-susceptible and ceftazidime-nonsusceptible pathogens in both the mMITT and CE populations, and were also similar to those for meropenem recipients [secondary endpoints] (Fig. 2b) [59].
Per-pathogen favourable microbiological response rates at TOC (secondary endpoint) for ceftazidime-avibactam and meropenem recipients, respectively, in the mMITT population were 62.7 and 74.6% for K. pneumoniae (n = 59 and 71; BGD − 11.9%; 95% CI − 27.76 to 4.03) and 37.9 and 38.3% for P. aeruginosa (n = 58 and 47; BGD − 0.4%; 95% CI − 19.01 to 17.98) [59]. In the extended microbiologically evaluable population, the corresponding rates were 78.4 and 79.6% for K. pneumoniae (n = 37 and 49; BGD − 1.2%; 95% CI − 19.60 to 15.96) and 42.9 and 40.0% for P. aeruginosa (n = 42 and 35; BGD 2.9%; 95% CI − 19.13 to 24.32) [59].
All-cause mortality at day 28 in the ceftazidime-avibactam and meropenem groups was 8.4 vs. 7.3% in the cMITT population and 4.7 vs. 3.3% in the CE population (secondary endpoints) [59].
4.4 Infections Due to Aerobic Gram-Negative Bacteria in Patients with Limited Treatment Options
Evidence in support of the efficacy of ceftazidime-avibactam in the treatment of infections caused by aerobic Gram-negative organisms in adult patients with limited treatment options is drawn from a variety of sources. Firstly, the vast amount of experience with ceftazidime used alone has shown that this third-generation cephalosporin is a very effective antimicrobial agent in the treatment of a variety of bacterial infections, particularly those caused by aerobic Gram-negative organisms [20]. Furthermore, as demonstrated in in vitro studies, the addition of avibactam to ceftazidime greatly increases the activity of ceftazidime, with the MIC90s of ceftazidime-avibactam against Enterobacteriaceae and P. aeruginosa reduced 128- and 8-fold, respectively, relative to ceftazidime alone (see Sect. 2.2). In vitro data has shown that the addition of avibactam broadens the spectrum of activity of ceftazidime to encompass many ESBL-, AmpC-, KPC- and OXA-48-producing isolates, including MDR organisms (see Sect. 2.2) [12,13,14,15,16,17]. Animal data support the findings from in vitro studies (see Sect. 2.3).
Phase III clinical data from trials in patients with a range of infection types provide further support for the utility of ceftazidime-avibactam in this indication. The pathogen-directed REPRISE trial (Sect. 4.1.2) in patients with cUTI or cIAI provided evidence that ceftazidime-avibactam has good efficacy in the treatment of infections caused by ceftazidime-nonsusceptible Gram-negative bacteria, similar to the efficacy of alternative best available therapy (predominantly carbapenems). Subgroup analyses in other phase III trials in patients with cUTI (RECAPTURE; Sect. 4.1.1), cIAI (RECLAIM; Sects. 4.2.1 and 4.2.2) and HAP including VAP (REPROVE; Sect. 4.3) provided further supportive evidence that ceftazidime-avibactam is efficacious in the treatment of infections caused by ceftazidime-nonsusceptible as well as ceftazidime-susceptible Gram-negative organisms. The efficacy of ceftazidime-avibactam against MDR Enterobacteriaceae and P. aeruginosa isolates was evaluated in a pooled analysis of data across the RECAPTURE-1 and -2, RECLAIM-1 and -2 and REPRISE trials (available as an abstract plus poster) [60]. Across the five trials, 876 (43%) of 2019 mMITT qualifying baseline Enterobacteriaceae and P. aeruginosa isolates were MDR (defined as resistant to ≥ 3 categories of antimicrobials). For MDR pathogens, the favourable per-pathogen response rates at the TOC visit in the ceftazidime-avibactam and comparator groups were 76.7% (309/403) vs. 69.0% (292/423) for all Enterobacteriaceae and 71.0% (22/31) vs. 78.9% (15/19) for P. aeruginosa isolates [60].
Analyses of the PK-PD relationship for ceftazidime-avibactam were also found to support the use of ceftazidime-avibactam in the treatment of infections caused by aerobic Gram-negative organisms in adult patients with limited treatment options, whereby the ceftazidime %fT > MIC of ceftazidime-avibactam and the avibactam %fT > CT are applicable predictors of ceftazidime-avibactam antimicrobial activity (Sect. 2.5) [11, 18].
5 Tolerability
Ceftazidime-avibactam was generally well tolerated in patients with cUTI, cIAI or HAP (including VAP) in clinical trials, with most adverse events being of mild to moderate intensity [55,56,57]. Overall, the safety and tolerability profile of ceftazidime-avibactam was consistent with that established for ceftazidime alone, and was generally typical of an injectable cephalosporin. The most commonly reported adverse reactions (occurring in ≥ 5% of patients) among ceftazidime-avibactam recipients (n = 2024) across seven phase II and phase III clinical trials were positive direct Coombs test, nausea and diarrhoea [18]. In phase III trials the frequencies of severe or serious adverse events or adverse events leading to treatment discontinuation or leading to death among ceftazidime-avibactam recipients were generally low and similar to the frequencies among patients treated with comparators [55,56,57,58,59]. No deaths in the phase III trials were considered related to the study drug (ceftazidime-avibactam or comparator).
The most commonly reported adverse event among ceftazidime-avibactam recipients in clinical trials was a positive direct Coombes test, with seroconversion (i.e. a negative test at baseline with a positive test post-baseline) occurring in 3.2–20.8% of evaluable ceftazidime-avibactam recipients across the phase III trials [18]. There was no evidence of haemolysis in ceftazidime-avibactam recipients who experienced seroconversion; however, haemolytic anaemia should be investigated as a possibility in patients experiencing anaemia during or after ceftazidime-avibactam treatment [18].
As is common for systemic antibacterial drugs, cases of Clostridium difficile-associated diarrhoea (CDAD) have occurred in patients treated with ceftazidime-avibactam [18]. CDAD can range from mild illness to a life-threatening condition. In patients presenting with diarrhoea during or after treatment with ceftazidime-avibactam a diagnosis of CDAD (and possible discontinuation of ceftazidime-avibactam) should be considered [18]. C. difficile colitis was reported in two (0.4%) ceftazidime-avibactam recipients (and no doripenem recipients) in the RECAPTURE trials [55] and in one patient in each treatment group in RECLAIM-1 and -2 [57]. Both cases in RECLAIM were toxin-positive but were considered to be non-serious and were moderate in intensity.
Renal impairment can affect ceftazidime and avibactam pharmacokinetics, causing decreased drug clearance and increased drug exposure (see Sect. 3). There have been occasional reports of neurological sequelae (e.g. tremor, myoclonus, non-convulsive status epilepticus, convulsion, encephalopathy and coma) occurring in patients with renal impairment who received ceftazidime without dose reduction [18]. Local prescribing information should be consulted for full details on ceftazidime-avibactam dosage reduction requirements in patients with impaired renal function.
6 Dosage and Administration
Ceftazidime-avibactam is approved in the EU for the treatment of adult patients with cUTI (including pyelonephritis), cIAI, HAP (including VAP) and other infections due to aerobic Gram-negative bacteria when there are limited treatment options [18]. When Gram-positive pathogens are known or suspected to be contributing to the infectious process, ceftazidime-avibactam is to be used in combination with an antibacterial agent active against such organisms. Similarly, when anaerobic pathogens are known or suspected to be contributing to the infectious process (particularly in cIAI), ceftazidime-avibactam is to be used in combination with metronidazole [18].
In the EU, the recommended ceftazidime-avibactam dosage for patients with a Cockcroft–Gault formula-estimated CLCR ≥ 51 mL/min is 2.5 g (i.e. ceftazidime 2 g, avibactam 0.5 g) once every 8 h, administered intravenously with an infusion time of 2 h in an infusion volume of 100 mL [18]. The recommended duration of treatment varies based on the indication for which ceftazidime-avibactam is used [18].
Due to the effects of renal impairment on ceftazidime and avibactam pharmacokinetics (see Sect. 3), the ceftazidime-avibactam dosage is to be reduced in patients with estimated CLCR ≤ 50 mL/min, with the recommended reductions dependent on the degree of renal impairment. Local prescribing information should be consulted for full details. It is recommended that the CLCR of patients with renal impairment is closely monitored during treatment with ceftazidime-avibactam, particularly given that CLCR can change quickly in some patients [18]. Also, the potential adverse effects on renal function of concurrent treatment with nephrotoxic medicinal products (e.g. aminoglycosides or potent diuretics such as furosemide) and high doses of cephalosporins (such as ceftazidime) should be considered [18]. In patients on haemodialysis, ceftazidime-avibactam dosing on dialysis days should occur after completion of the haemodialysis [18].
Prior to commencing treatment with ceftazidime-avibactam, any possible history of hypersensitivity reactions to ceftazidime or other cephalosporins or any other β-lactam antibacterial agent should be established [18]. Ceftazidime-avibactam is contraindicated in any patient with known hypersensitivity to any cephalosporin antibacterial agent or known severe hypersensitivity to any other type of β-lactam antibacterial agent [18].
Co-administration of ceftazidime-avibactam and chloramphenicol should be avoided given the possibility of drug antagonism, which has been observed between chloramphenicol and cephalosporins (including ceftazidime) in vitro [18]. Co-administration of ceftazidime-avibactam with OAT1/OAT3 inhibitors such as probenecid should also be avoided (see Sect. 3).
Local prescribing information should be consulted for full details regarding the use of ceftazidime-avibactam, including further information on contraindications, warnings and precautions.
7 Place of Ceftazidime-Avibactam in the Treatment of Serious Gram-Negative Bacterial Infections
Antimicrobial therapies for the treatment of infections caused by drug-resistant Gram-negative organisms include carbapenems (e.g. meropenem, imipenem, ertapenem), later-generation cephalosporins (e.g. ceftazidime, cefepime), tigecycline, β-lactam/β-lactamase inhibitor combinations (e.g. piperacillin-tazobactam, ceftolozane-tazobactam, ceftazidime-avibactam), monobactams (e.g. aztreonam), fluoroquinolones (e.g. levofloxacin), aminoglycosides (e.g. amikacin) and polymixins (e.g. colistin) [61,62,63,64]. Carbapenems (either as monotherapy, or in combination with other antimicrobials) have been the drugs of choice for the treatment of infections known or suspected to involve drug-resistant organisms [3, 5, 61, 65]. However, the growing prevalence of MDR organisms and, in particular, the spread of carbapenem resistance among Gram-negative pathogens is of significant concern and has highlighted the need for new antimicrobial agents [1, 6]. In this context, ceftazidime-avibactam, comprised of the extended-spectrum cephalosporin ceftazidime and the novel β-lactamase inhibitor avibactam, was developed as an alternative treatment for serious infections caused by Gram-negative organisms. In the EU, ceftazidime-avibactam is approved for the treatment of adult patients with cUTI (including pyelonephritis), cIAI, HAP (including VAP) and other infections due to aerobic Gram-negative bacteria when there are limited treatment options.
Ceftazidime is an established third-generation cephalosporin with a broad spectrum of antimicrobial activity, including against P. aeruginosa. It has been an effective agent in the treatment of a range of bacterial infections. However, the spread of cephalosporin resistance, through mechanisms such as the production of ESBLs, has somewhat limited its utility in the treatment of serious infections. Combining ceftazidime with the novel β-lactamase inhibitor avibactam improves its in vitro activity against a range of β-lactamase-producing aerobic Gram-negative pathogens. Overall, 99.5% of Enterobacteriaceae and 92.0% of P. aeruginosa isolates collected in the INFORM global surveillance program in 2012–2014 were found to be susceptible to ceftazidime-avibactam (Sect. 2.2). Moreover, the addition of avibactam has been shown to restore the in vitro activity of ceftazidime against many ESBL-, AmpC-, KPC- and OXA-48-producing Enterobacteriaceae and ceftazidime-nonsusceptible P. aeruginosa isolates.
The clinical efficacy of intravenous ceftazidime-avibactam in the treatment of cUTI, cIAI and HAP (including VAP) in adults was demonstrated in pivotal phase III non-inferiority trials (all versus carbapenem comparators), where ceftazidime-avibactam treatment was associated with high response rates (Sect. 4). Ceftazidime-avibactam was non-inferior to doripenem in the treatment of hospitalized adults with cUTI (including pyelonephritis) in the RECAPTURE trials, based on primary endpoint analyses (Sect. 4.1.1). Furthermore, ceftazidime-avibactam was superior to doripenem (at the 5% significance level) based on the microbiological response rate at the TOC visit. Doripenem was considered an appropriate comparator agent for the demonstration of efficacy in cUTI [55], but it should be noted that EU approval for doripenem was withdrawn in 2014. Ceftazidime-avibactam plus metronidazole was non-inferior to meropenem in the treatment of hospitalized adults with cIAI, based on clinical cure rates at the TOC visit in the RECLAIM-1 and -2 trials (Sect. 4.2.1) as well as in the RECLAIM-3 trial in Asian populations (Sect. 4.2.2). As demonstrated in the pivotal REPROVE trial (Sect. 4.3), ceftazidime-avibactam was also non-inferior to meropenem in the treatment of adult patients with HAP (including VAP), with REPROVE being the first randomized controlled trial to demonstrate non-inferiority of a new antimicrobial therapy versus a carbapenem targeting Gram-negative pathogens in this setting.
Phase III clinical trial data also support the findings from in vitro studies that found that ceftazidime-avibactam could be an effective treatment against ceftazidime-nonsusceptible aerobic Gram-negative pathogens. Although not powered for inferential statistical analysis and with the limitation of an open-label design, the REPRISE trial in adult patients with cUTI or cIAI caused by ceftazidime-nonsusceptible Enterobacteriaceae or P. aeruginosa showed that ceftazidime-avibactam treatment was associated with a similar clinical cure rate to that of alternative best available therapy (predominantly carbapenems) (Sect. 4.1.2). Subgroup analyses in other phase III trials in patients with serious bacterial infections provided further supportive evidence that ceftazidime-avibactam is efficacious in the treatment of infections caused by ceftazidime-nonsusceptible aerobic Gram-negative organisms (Sect. 4.4).
One potential limitation of the pivotal trials was the low numbers of more critically ill patients that were included, with patients with an APACHE II score > 30 excluded from the RECLAIM and REPROVE trials. A phase I study (NCT02822950) investigating the pharmacokinetics of ceftazidime-avibactam in critically ill patients (APACHE II score ≥ 15) is planned and will be of interest, particularly given the potential role of ceftazidime-avibactam in the critical care setting.
Intravenous ceftazidime-avibactam was generally well tolerated in patients in the phase III trials, with most adverse events being of mild to moderate intensity (Sect. 5). Overall, the safety and tolerability profile of ceftazidime-avibactam was consistent with that of ceftazidime alone, and was generally typical of the injectable cephalosporins.
The pharmacokinetic properties of ceftazidime and avibactam are well matched for use in combination, with the two agents having similar volumes of distribution, with both showing low plasma protein binding and undergoing renal elimination with short plasma half-lives (Sect. 3) [11]. Furthermore, no drug interaction is observed between ceftazidime and avibactam, nor between ceftazidime-avibactam and metronidazole. Indeed, ceftazidime-avibactam appears to have a low potential for drug-drug interactions (Sect. 3), an important finding given that in the clinical setting ceftazidime-avibactam is likely to commonly be co-administered with other drugs [48, 51]. Ceftazidime-avibactam has no dosage adjustment requirements based on gender, age, race or hepatic impairment, but dosage reductions are required for patients with CLCR ≤ 50 mL/min (Sects. 3 and 6).
Based on available data, the potential for the selection of resistance to ceftazidime-avibactam appears to be relatively low (Sect. 2.4). However, as with all new antibiotics, surveillance for the potential emergence of resistance will be important.
In conclusion, the treatment of serious infections remains challenging, particularly given the growing prevalence of ESBL-producing, KPC-producing and MDR organisms [61, 62]. Treatment decisions require consideration of several factors, including the likely pathogen (or pathogens), local antimicrobial resistance patterns, patient-specific factors (e.g. site of infection, presence of comorbidities, prior antibiotic therapy, drug allergies), drug properties (e.g. the drug safety and tolerability profile, potential for drug-drug interactions), pharmacoeconomic considerations, and antimicrobial stewardship considerations to reduce the development or spread of resistance. Further treatment decisions will be guided by the clinical and microbiological response of the patient and the identification of any isolated pathogen(s), including information on antimicrobial susceptibility/resistance. Within this context, ceftazidime-avibactam has been shown to be efficacious and generally well tolerated in the treatment of serious infections caused by aerobic Gram-negative bacteria, including cUTI, cIAI and HAP (including VAP). The clinical trial data support and complement in vitro studies that show that ceftazidime-avibactam has excellent in vitro activity against many important Gram-negative pathogens, including many ESBL-, AmpC-, KPC- and OXA-48-producing Enterobacteriaceae and drug-resistant P. aeruginosa isolates. Ceftazidime-avibactam is approved in the EU for the treatment of adults with cUTI, cIAI or HAP and other infections caused by aerobic Gram-negative bacteria in adult patients with limited treatment options. Thus, this drug combination represents a valuable new option for the treatment of these difficult-to-treat infections, and will be a useful addition to available antimicrobial treatment options given the serious and growing threat to global public health presented by drug-resistant bacteria.
Data Selection Ceftazidime-Avibactam: 161 records identified
Duplicates removed | 9 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 59 |
Excluded during writing (e.g. not randomized trials; review; duplicate data; small patient number; phase I/II trials) | 28 |
Cited efficacy/tolerability articles | 12 |
Cited articles not efficacy/tolerability | 53 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were Ceftazidime, avibactam, AVE-1330, Avycaz, Zavicefta. Records were limited to those in English language. Searches last updated 12 March 2018 | |
References
World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. 2017. http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1. Accessed 16 Mar 2018.
Tängdén T, Giske CG. Global dissemination of extensively drug-resistant carbapenemase-producing Enterobacteriaceae: clinical perspectives on detection, treatment and infection control. J Intern Med. 2015;277(5):501–12.
Zowawi HM, Harris PNA, Roberts MJ, et al. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat Rev Urol. 2015;12(10):570–84.
Bush K. Bench-to-bedside review: the role of β-lactamases in antibiotic-resistant Gram-negative infections. Crit Care. 2010;14(3):224.
Pitout JDD. Infections with extended-spectrum β-lactamase-producing Enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs. 2010;70(3):313–33.
Cantón R, Akóva M, Carmeli Y, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect. 2012;18(5):413–31.
Bebrone C, Lassaux P, Vercheval L, et al. Current challenges in antimicrobial chemotherapy: focus on β-lactamase inhibition. Drugs. 2010;70(6):651–79.
Bonnefoy A, Dupuis-Hamelin C, Steier V, et al. In vitro activity of AVE1330A, an innovative broad-spectrum non-β-lactam β-lactamase inhibitor. J Antimicrob Chemother. 2004;54(2):410–7.
Ehmann DE, Jahić H, Ross PL, et al. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc Natl Acad Sci USA. 2012;109(29):11663–8.
Stachyra T, Péchereau M-C, Bruneau J-M, et al. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-β-lactam β-lactamase inhibitor. Antimicrob Agents Chemother. 2010;54(12):5132–8.
European Medicines Agency. European public assessment report: Zavicefta (ceftazidime/avibactam). 2016. http://www.ema.europa.eu. Accessed 16 Mar 2018.
Berkhout J, Melchers MJ, van Mil AC, et al. In vitro activity of ceftazidime-avibactam combination in in vitro checkerboard assays. Antimicrob Agents Chemother. 2015;59(2):1138–44.
Lagacé-Wiens PRS, Tailor F, Simner P, et al. Activity of NXL104 in combination with β-lactams against genetically characterized Escherichia coli and Klebsiella pneumoniae isolates producing class A extended-spectrum β-lactamases and class C β-lactamases. Antimicrob Agents Chemother. 2011;55(5):2434–7.
Levasseur P, Girard A-M, Miossec C, et al. In vitro antibacterial activity of the ceftazidime-avibactam combination against Enterobacteriaceae, including strains with well-characterized β-lactamases. Antimicrob Agents Chemother. 2015;59(4):1931–4.
Livermore DM, Mushtaq S, Warner M, et al. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2011;55(1):390–4.
Karlowsky JA, Biedenbach DJ, Kazmierczak KM, et al. Activity of ceftazidime-avibactam against extended-spectrum- and AmpC β-lactamase-producing Enterobacteriaceae collected in the INFORM global surveillance study from 2012 to 2014. Antimicrob Agents Chemother. 2016;60(5):2849–57.
Kazmierczak KM, Biedenbach DJ, Hackel M, et al. Global dissemination of bla KPC into bacterial species beyond Klebsiella pneumoniae and in vitro susceptibility to ceftazidime-avibactam and aztreonam-avibactam. Antimicrob Agents Chemother. 2016;60(8):4490–500.
European Medicines Agency. Zavicefta: summary of product characteristics. 2018. http://www.ema.europa.eu. Accessed 16 Mar 2018.
US FDA. Avycaz (ceftazidime and avibactam) for injection, for intravenous use: US prescribing information. 2018. https://www.accessdata.fda.gov. Accessed 16 Mar 2018.
Rains CP, Bryson HM, Peters DH. Ceftazidime: an update of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1995;49(4):577–617.
Lahiri SD, Mangani S, Durand-Reville T, et al. Structural insight into potent broad-spectrum inhibition with reversible recyclization mechanism: avibactam in complex with CTX-M-15 and Pseudomonas aeruginosa AmpC β-lactamases. Antimicrob Agents Chemother. 2013;57(6):2496–505.
Ehmann DE, Jahić H, Ross PL, et al. Kinetics of avibactam inhibition against class A, C, and D β-lactamases. J Biol Chem. 2013;288(39):27960–71.
Papp-Wallace KM, Bajaksouzian S, Abdelhamed AM, et al. Activities of ceftazidime, ceftaroline, and aztreonam alone and combined with avibactam against isogenic Escherichia coli strains expressing selected single β-lactamases. Diagn Microbiol Infect Dis. 2015;82(1):65–9.
Aktaş Z, Kayacan C, Oncul O. In vitro activity of avibactam (NXL104) in combination with β-lactams against Gram-negative bacteria, including OXA-48 β-lactamase-producing Klebsiella pneumoniae. Int J Antimicrob Agents. 2012;39(1):86–9.
de Jonge BLM, Karlowsky JA, Kazmierczak KM, et al. In vitro susceptibility to ceftazidime-avibactam of carbapenem-nonsusceptible Enterobacteriaceae isolates collected during the INFORM global surveillance study (2012 to 2014). Antimicrob Agents Chemother. 2016;60(5):3163–9.
Nichols WW, de Jonge BLM, Kazmierczak KM, et al. In vitro susceptibility of global surveillance isolates of Pseudomonas aeruginosa to ceftazidime-avibactam (INFORM 2012 to 2014). Antimicrob Agents Chemother. 2016;60(8):4743–9.
Testa R, Cantón R, Giani T, et al. In vitro activity of ceftazidime, ceftaroline and aztreonam alone and in combination with avibactam against European Gram-negative and Gram-positive clinical isolates. Int J Antimicrob Agents. 2015;45(6):641–6.
Buehrle DJ, Shields RK, Chen L, et al. Evaluation of the in vitro activity of ceftazidime-avibactam and ceftolozane-tazobactam against meropenem-resistant Pseudomonas aeruginosa isolates. Antimicrob Agents Chemother. 2016;60(5):3227–31.
Alatoom A, Elsayed H, Lawlor K, et al. Comparison of antimicrobial activity between ceftolozane-tazobactam and ceftazidime-avibactam against multidrug-resistant isolates of Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Int J Infect Dis. 2017;62:39–43.
Scott LJ. Ceftolozane/tazobactam: a review in complicated intra-abdominal and urinary tract infections. Drugs. 2016;76(2):231–42.
Crandon JL, Schuck VJ, Banevicius MA, et al. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2012;56(12):6137–46.
MacVane SH, Crandon JL, Nichols WW, et al. In vivo efficacy of humanized exposures of ceftazidime-avibactam in comparison with ceftazidime against contemporary Enterobacteriaceae isolates. Antimicrob Agents Chemother. 2014;58(11):6913–9.
Housman ST, Crandon JL, Nichols WW, et al. Efficacies of ceftazidime-avibactam and ceftazidime against Pseudomonas aeruginosa in a murine lung infection model. Antimicrob Agents Chemother. 2014;58(3):1365–71.
Levasseur P, Girard A-M, Lavallade L, et al. Efficacy of a ceftazidime-avibactam combination in a murine model of septicemia caused by Enterobacteriaceae species producing AmpC or extended-spectrum β-lactamases. Antimicrob Agents Chemother. 2014;58(11):6490–5.
Endimiani A, Hujer KM, Hujer AM, et al. Evaluation of ceftazidime and NXL104 in two murine models of infection due to KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2011;55(1):82–5.
Lahiri SD, Walkup GK, Whiteaker JD, et al. Selection and molecular characterization of ceftazidime/avibactam-resistant mutants in Pseudomonas aeruginosa strains containing derepressed AmpC. J Antimicrob Chemother. 2015;70(6):1650–8.
Livermore DM, Mushtaq S, Barker K, et al. Characterization of β-lactamase and porin mutants of Enterobacteriaceae selected with ceftaroline + avibactam (NXL104). J Antimicrob Chemother. 2012;67(6):1354–8.
Livermore DM, Warner M, Jamrozy D, et al. In vitro selection of ceftazidime-avibactam resistance in Enterobacteriaceae with KPC-3 carbapenemase. Antimicrob Agents Chemother. 2015;59(9):5324–30.
Haidar G, Clancy CJ, Shields RK, et al. Mutations in bla KPC-3 that confer ceftazidime-avibactam resistance encode novel KPC-3 variants that function as extended-spectrum β-lactamases. Antimicrob Agents Chemother. 2017;61(5):e02534-16.
Shields RK, Chen L, Cheng S, et al. Emergence of ceftazidime-avibactam resistance due to plasmid-borne bla KPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother. 2017;61(3):e02097-16.
Winkler ML, Papp-Wallace KM, Hujer AM, et al. Unexpected challenges in treating multidrug-resistant Gram-negative bacteria: resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2015;59(2):1020–9.
Pagès J-M, Peslier S, Keating TA, et al. Role of the outer membrane and porins in susceptibility of β-lactamase-producing Enterobacteriaceae to ceftazidime-avibactam. Antimicrob Agents Chemother. 2016;60(3):1349–59.
Shen Z, Ding B, Ye M, et al. High ceftazidime hydrolysis activity and porin OmpK35 deficiency contribute to the decreased susceptibility to ceftazidime/avibactam in KPC-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2017;72(7):1930–6.
Muller AE, Punt N, Mouton JW. Optimal exposures of ceftazidime predict the probability of microbiological and clinical outcome in the treatment of nosocomial pneumonia. J Antimicrob Chemother. 2013;68(4):900–6.
Berkhout J, Melchers MJ, van Mil AC, et al. Pharmacodynamics of ceftazidime and avibactam in neutropenic mice with thigh or lung infection. Antimicrob Agents Chemother. 2016;60(1):368–75.
Li J, Zhou D, Nichols WW, et al. PK/PD target attainment analyses and assessment of dose adjustments for renal insufficiency for ceftazidime-avibactam (CAZ-AVI) in patients with complicated intra-abdominal infection (cIAI), complicated urinary tract infection (cUTI) or nosocomial pneumonia (NP) [abstract no. R6302]. In: American Association of Pharmaceutical Scientists (AAPS) Annual Meeting and Exposition. 2015.
Merdjan H, Rangaraju M, Tarral A. Safety and pharmacokinetics of single and multiple ascending doses of avibactam alone and in combination with ceftazidime in healthy male volunteers: results of two randomized, placebo-controlled studies. Clin Drug Investig. 2015;35(5):307–17.
Das S, Li J, Armstrong J, et al. Randomized pharmacokinetic and drug-drug interaction studies of ceftazidime, avibactam, and metronidazole in healthy subjects. Pharmacol Res Perspect. 2015;3(5):e00172.
Nicolau DP, Siew L, Armstrong J, et al. Phase 1 study assessing the steady-state concentration of ceftazidime and avibactam in plasma and epithelial lining fluid following two dosing regimens. J Antimicrob Chemother. 2015;70(10):2862–9.
Dimelow R, Wright J, Macpherson M, et al. Population pharmacokinetic modelling of ceftazidime and avibactam in the plasma and epithelial lining fluid of healthy volunteers [abstract and poster no. EP0350]. In: 27th European Congress of Clinical Microbiology and Infectious Diseases. 2017.
Vishwanathan K, Mair S, Gupta A, et al. Assessment of the mass balance recovery and metabolite profile of avibactam in humans and in vitro drug-drug interaction potential. Drug Metab Dispos. 2014;42(5):932–42.
Welage LS, Schultz RW, Schentag JJ. Pharmacokinetics of ceftazidime in patients with renal insufficiency. Antimicrob Agents Chemother. 1984;25(2):201–4.
Merdjan H, Tarral A, Das S, et al. Phase 1 study assessing the pharmacokinetic profile and safety of avibactam in patients with renal impairment. J Clin Pharmacol. 2017;57(2):211–8.
Tarral A, Merdjan H. Effect of age and sex on the pharmacokinetics and safety of avibactam in healthy volunteers. Clin Ther. 2015;37(4):877–86.
Wagenlehner FM, Sobel JD, Newell P, et al. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis. 2016;63(6):754–62.
Carmeli Y, Armstrong J, Laud PJ, et al. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis. 2016;16(6):661–73.
Mazuski JE, Gasink LB, Armstrong J, et al. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis. 2016;62(11):1380–9.
Qin X, Tran BG, Kim MJ, et al. A randomised, double-blind, phase 3 study comparing the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem for complicated intra-abdominal infections in hospitalised adults in Asia. Int J Antimicrob Agents. 2017;49(5):579–88.
Torres A, Zhong N, Pachl J, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis. 2017;18(3):285–95.
Stone GG, Gasink L, Newell P, et al. Efficacy of ceftazidime-avibactam against multi-drug resistant Enterobacteriaceae and Pseudomonas aeruginosa from the phase 3 clinical trial program [abstract no. 2044 plus poster]. In: IDWeek. 2016.
Golan Y. Empiric therapy for hospital-acquired, Gram-negative complicated intra-abdominal infection and complicated urinary tract infections: a systematic literature review of current and emerging treatment options. BMC Infect Dis. 2015;15:313.
Sartelli M, Weber DG, Ruppé E, et al. Antimicrobials: a global alliance for optimizing their rational use in intra-abdominal infections (AGORA). World J Emerg Surg. 2016;11:33.
Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–111.
European Association of Urology. Urological infections guidelines. 2017. http://uroweb.org/guideline/urological-infections/. Accessed 16 Mar 2018.
Arizpe A, Reveles KR, Patel SD, et al. Updates in the management of cephalosporin-resistant Gram-negative bacteria. Curr Infect Dis Rep. 2016;18(12):39.
Acknowledgements
During the peer review process, the manufacturer of ceftazidime-avibactam was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Matt Shirley is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: T. Cai, Department of Urology, Santa Chiara Regional Hospital, Trento, Italy; G. Poulakou, Fourth Department of Internal Medicine, Athens National and Kapodistrian University, School of Medicine, Attikon University General Hospital, Athens, Greece; P.M. Tulkens, Louvain Drug Research Institute, Université catholique de Louvain, Brussels, Belgium; R. Zaragoza, Intensive Care Unit, Sepsis Team, Hospital Universitario Dr. Peset, Valencia, Spain.
Rights and permissions
About this article
Cite this article
Shirley, M. Ceftazidime-Avibactam: A Review in the Treatment of Serious Gram-Negative Bacterial Infections. Drugs 78, 675–692 (2018). https://doi.org/10.1007/s40265-018-0902-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0902-x