Summary
Abstract
Raltegravir (Isentress®), an integrase inhibitor, inhibits the insertion of HIV-1 complementary DNA into the host genome. It is indicated in combination with other antiretroviral therapy (ART) agents for the treatment of HIV-1 infection in treatment-experienced adult patients who have evidence of viral replication and HIV-1 strains resistant to multiple ART agents. It is the first of a new class of ART agents to be approved that, as a result of a different mechanism of action to other ART agents, has good activity against multidrug-resistant HIV-1 strains.
In clinical trials in treatment-experienced patients with HIV-1 infection and evidence of viral replication, the addition of oral raltegravir to an optimized background therapy (OBT) regimen improved virological and immunological responses at 16 and 48 weeks to a greater extent than placebo plus OBT. Raltegravir therapy was generally well tolerated, with a similar incidence of mild to moderate adverse events in the treatment and placebo arms. The introduction of integrase inhibitors extends the options available for managing treatment-experienced patients with multiple-drug-resistant HIV-1 infection. Results to date suggest that the combination of raltegravir and OBT will be a valuable treatment option for this difficult-to-treat patient group.
Pharmacological Properties
Raltegravir is a selective inhibitor of integrase, an HIV-1-specific enzyme that is responsible for the insertion of viral complimentary DNA into the host genome. In in vitro studies, the 95% inhibitory concentration (IC95) of raltegravir in human T-lymphoid cells infected with a laboratory strain of HIV-1 was 31 nmol/L and ranged from 6 to 50 nmol/L in human peripheral blood mono-nuclear cells, infected with clinical isolates of HIV-1, including strains resistant to other classes of ART. Specific mutations within the HIV-1 integrase gene (predominantly at Q148, N155 or Y143) are correlated with virological resistance to raltegravir. Patients with higher baseline levels of HIV-1 RNA and less active OBT regimens are at the highest risk of integrase mutations arising during treatment.
Oral raltegravir is rapidly absorbed, with a mean maximum plasma concentration reached at a median time of ≈1 hour. The mean plasma concentration of raltegravir at the end of a 12-hour dose administration interval after a 400 mg dose exceeded the IC95 against HIV-1 in vitro. Raltegravir is metabolized in humans through uridine diphosphate-glucuronosyltransferase-mediated glucuronidation in the liver, and is eliminated in faeces and urine. The mean elimination half-life of raltegravir is 7–12 hours. Raltegravir does not significantly affect the activity of the cytochrome P450 system and therefore is not expected to interact with other ART agents that are substrates for these enzymes.
Therapeutic Efficacy
In the two phase III BENCHMRK trials (n = 699), orally administered raltegravir 400 mg twice daily plus OBT improved both virological and immunological outcomes in treatment-experienced patients with HIV-1 infection, evidence of viral replication and HIV-1 strains resistant to multiple ART agents. At 16 weeks in the individual trials and at 48 weeks in the combined analysis, raltegravir plus OBT reduced HIV-1 RNA levels to <400 copies/mL (primary endpoint) and <50 copies/mL in a significantly greater number of patients and significantly increased CD4+ cell counts from baseline when compared with placebo plus OBT. Moreover, prespecified subgroup analyses indicated that in the BENCHMRK trials, the greater efficacy of raltegravir plus OBT compared with placebo plus OBT was maintained in patients with baseline characteristics that typically predict a poor response to ART therapy, for example, higher HIV-1 RNA levels, lower CD4+ cell counts and less active OBT regimens.
Tolerability
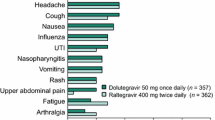
Raltegravir was generally well tolerated when used in combination with OBT regimens in treatment-experienced patients with HIV-1 infection in trials of up to 48 weeks’ duration. The majority of adverse events were mild to moderate in severity, and of similar incidence in the raltegravir plus OBT and placebo plus OBT treatment arms; treatment-related discontinuations were uncommon. The most common drug-related adverse events reported were diarrhoea, headache, nausea and fatigue.






Similar content being viewed by others
References
Joint United Nations Programme on HIV/AIDS. 2008 report on the global AIDS epidemic [online]. Available from URL: http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp [Accessed 2008 Nov 26]
WHO, UNAIDS, UNICEF. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report 2008 [online]. Available from URL: http://www.who.int/hiv/pub/towards_universal_access_report_2008.pdf [Accessed 2008 Nov 26]
WHO. Global HIV prevalence has levelled off: improvements in surveillance increase understanding of the epidemic, resulting in substantial revisions to estimates [online]. Available from URL: http://www.who.int/mediacentre/news/releases/2007/pr61/en/print.html [Accessed 2008 Nov 26]
Shet A, Berry L, Mohri H, et al. Tracking the prevalence of transmitted antiretroviral drug-resistant HIV-1. J Aquir Immune Defic Syndr 2006; 41(4): 439–46
Dybul M, Fauci AS, Bartlett JG, et al. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents: recommendations of the Panel on Clinical Practices for Treatment of HIV. MMWR Recomm Rep 2002 May 17; 51(RR-7): 1–55
Richman D, Morton S, Wrin T, et al. The prevalence of antiretroviral drug resistance in the United States. AIDS 2004; 18(10): 1393–401
Pommier Y, Johnson AA, Marchand C. Integrase inhibitors to treat HIV/AIDS. Nat Rev Drug Discov 2005; 4(3): 236–48
Palmisano L. Role of integrase inhibitors in the treatment of HIV disease: expert review of anti-infective therapy. Exp Rev Anti-Infect Ther 2007; 5(1): 67–75
Croxtall JD, Lyseng-Williamson KA, Perry CM. Raltegravir. Drugs 2008; 68(1): 131–8
Summa V, Petrocchi A, Bonelli F, et al. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J Med Chem 2008; 51(18): 5843–55
Marinello J, Marchand C, Mott BT, et al. Comparison of raltegravir and elvitegravir on HIV-1 integrase catalytic reactions and on a series of drug-resistant integrase mutants. Biochemistry (Mosc) 2008; 47(36): 9345–54
Danovich R, Ke Y, Wan H, et al. Raltegravir has similar in vitro antiviral potency, clinical efficacy, and resistance patterns in B subtype and non-B subtype HIV-1 [abstract no. TUAA0302]. 17th International AIDS Conference; 2008 Aug 3–8; Mexico City
Roquebert B, Damond F, Collin G, et al. HIV-2 integrase gene polymorphism and phenotypic susceptibility of HIV-2 clinical isolates to the integrase inhibitors raltegravir and elvite-gravir in vitro. J Antimicrob Chemother 2008; 62(5): 914–20
Cooper DA, Steigbigel RT, Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med 2008; 359(4): 355–65
Charpentier C, Karmochkine M, Laureillard D, et al. Drug resistance profiles for the HIV integrase gene in patients failing raltegravir salvage therapy. HIV Med 2008; 9(9): 765–70
Malet I, Delelis O, Valantin MA, et al. Mutations associated with failure of raltegravir treatment affect integrase sensitivity to the inhibitor in vitro. Antimicrob Agents Chemother 2008; 52(4): 1351–8
Van Baelen K, Van Eygen V, Rondelez E, et al. Clade-specific HIV-1 integrase polymorphisms do not reduce raltegravir and elvitegravir phenotypic susceptibility. AIDS 2008; 22(14): 1877–80
Isentress (raltegravir): US prescribing information. Whitehouse Station (NJ): Merck and Co., Inc., 2009
European Medicines Agency. Raltegravir: summary of product characteristics [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/isentress/H-860-PI-en.pdf [Accessed 2009 May 20]
Rowley M. The discovery of raltegravir, an integrase inhibitor for the treatment of HIV infection. Prog Med Chem 2008; 46: 1–28
Evering TH, Markowitz M. Raltegravir (MK-0518): an integrase inhibitor for the treatment of HIV-1. Drugs Today 2007; 43(12): 865–77
Hazuda DJ, Felock P, Witmer M, et al. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science 2000; 287(5453): 646–50
Roquebert B, Blum L, Collin G, et al. Selection of the Q148R integrase inhibitor resistance mutation in a failing raltegravir containing regimen [letter]. AIDS 2008; 22(15): 2045–6
Hazuda DJ, Miller MD, Nguyen BY. Resistance to the HIV-integrase inhibitor raltegravir: analysis of protocol 005, a phase II study in patients with triple-class resistant HIV-1 infection [abstract no. 8]. Antiviral Therapy 2007 June 12–16; 12(5): S10
Garrett N, Xu L, Smit E, et al. Raltegravir treatment response in an HIV-2 infected patient: a case report [letter]. AIDS 2008 May 31; 22(9): 1091–2
Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med 2008; 359(4): 339–54
Iwamoto M, Kost JT, Mistry GC, et al. Raltegravir thorough QT/QTc study: a single supratherapeutic dose of raltegravir does not prolong the QTcF interval. J Clin Pharmacol 2008; 48(6): 726–33
Wenning LA, Petry A, Kost JT, et al. Pharmacokinetics of raltegravir in individuals with UGT1A1polymorphisms. Clin Pharmacol Ther. Epub 2009 Mar 11
Wenning L, Anderson M, Petry A, et al. Raltegravir (RAL) dose proportionality and effect of food [abstract no. H-1046]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Iwamoto M, Wenning LA, Petry AS, et al. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin Pharmacol Ther 2008; 83(2): 293–9
Kassahun K, McIntosh I, Cui D, et al. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the HIV-1 integrase enzyme. Drug Metab Dispos 2007; 35(9): 1657–63
Iwamoto M, Wenning LA, Mistry GC, et al. Atazanavir modestly increases plasma levels of raltegravir in healthy subjects. Clin Infect Dis 2008; 47(1): 137–40
Iwamoto M, Wenning LA, Petry AS, et al. Minimal effects of ritonavir and efavirenz on the pharmacokinetics of raltegravir. Antimicrob Agents Chemother 2008; 52(12): 4338–43
Iwamoto M, Kassahun K, Troyer MD, et al. Lack of a pharmacokinetic effect of raltegravir on midazolam: in vitro/in vivo correlation. J Clin Pharmacol 2008; 48(2): 209–14
Iwamoto M, Wenning, LA, Nguyen BY et al. Effects of omeprazole on plasma levels of raltegravir. Clin Infect Dis. Epub 2009 Jan 1
Markowitz M, Morales-Ramirez JO, Nguyen BY, et al. Anti-retroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J Acquir Immune Defic Syndr 2006; 43(5): 509–15
Iwamoto M, Hanley WD, Petry AS. Lack of a clinically important effect of moderate hepatic insufficiency and severe renal insufficiency on raltegravir pharmacokinetics. Antimicrob Agents Chemother 2009 May; 53(5): 1747–52
Wenning L, Hanley W, Brainard D, et al. Effect of rifampin, a potent inducer of drug metabolizing enzymes, on the pharmacokinetics of raltegravir. Antimicrob Agents Chemother. Epub 2009 May 11
Hanley W, Wenning L, Moreau A, et al. Effect of tipranavir + ritonavir on pharmacokinetics of raltegravir. Antimicrob Agents Chemother. Epub 2009 Apr 27
McKeage K, Perry C, Keam S. Darunavir: a review of its use in the management of HIV infection in adults. Drugs 2009; 69(4): 477–503
Markowitz M, Nguyen B-Y, Gotuzzo E, et al. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part combination therapy in treatment-naive patients with HIV-1 infection. J Acquir Immune Defic Syndr 2007; 46(2): 125–33
Markowitz M, Nguyen BY, Gotuzzo E, et al. Sustained antiretroviral efficacy of raltegravir as part of combination ART in treatment-naive HIV-1 infected patients: 96-week data [abstract no. TUAB0102]. 17th International AIDS Conference; 2008 Aug 3–8; Mexico City
Steigbigel R, Cooper D, Eron J, et al. 96-week results from BENCHMRK 1 and 2, phase III studies of raltegravir in patients failing ART with triple-class-resistant HIV [abstract no. K-105]. 16th Conference on Retroviruses and Opportunistic Infections; 2009 Feb 9–11; Montreal (QC)
Grinsztejn B, Nguyen BY, Katlama C, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet 2007; 369(9569): 1261–9
Yazdanpanah Y, Fagard C, Descamps D, et al. High rate of virologic success with raltegravir plus etravirine and darunavir/ritonavir in treatment-experienced patients with multidrug-resistant virus: results of the ANRS 139 TRIO trial [abstract no. THAB0406]. 17th International AIDS Conference; 2008 Aug 3–8; Mexico City
Harris M, Larsen G, Montaner JS. Outcomes of multidrug-resistant patients switched from enfuvirtide to raltegravir within a virologically suppressive regimen. AIDS 2008 Jun 19; 22(10): 1224–6
De Castro N, Braun J, Charreau I, et al. Switch from enfuvirtide (E) to raltegravir (R) in highly treatment-experienced HIV-1 infected patients: a randomized open-label non-inferiority trial (Easier — ANRS 138) [abstract no. 572]. 16th Conference on Retroviuses and Opportunistic Infections; 2009 Feb 8–11; Montreal (QC)
Cooper D, Steigbigel R, Lennox J, et al. Review of cancer incidence in raltegravir (RAL) clinical trials [abstract no. R-106]. 16th Conference on Retroviruses and Opportunistic Infections; 2009 Feb 8–11; Montreal (QC)
DHHS panel on antiretroviral guidelines for adults and adolescents: a working group of the Office of AIDS Research Advisory Council (OARAC). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents [online]. Available from URL: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf [Accessed 2008 Nov 26]
Hammer SM, Eron Jr JJ, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA 2008; 300(5): 555–70
Gazzard BG. British HIV Association guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Med 2008; 9(8): 563–608
European AIDS Clinical Society (EACS). Guidelines for the clinical management and treatment of HIV infected adults in Europe [online]. Available from URL: http://www.europeanaidsclinicalsociety.org/guidelinespdf/1_Treatment_of_HIV_Infected_Adults.pdf [Accessed 2009 Mar 26]
Palella FJ, Delaney K, Moorman A, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998; 338(13): 853–60
Hirsch MS, Gunthard HF, Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis 2008; 47(2): 266–85
Daar E. Emerging resistance profiles of newly approved antiretroviral drugs. Top HIV Med 2008; 16(4): 110–6
Hughes A, Barber T, Nelson M. New treatment options for HIV salvage patients: an overview of second generation PIs, NNRTIs, integrase inhibitors and CCR5 antagonists. J Infect 2008; 57(1): 1–10
Elbasha E, Szucs T, Chaudhary M, et al. Cost-effectiveness analysis of raltegravir in treatment-experienced HIV-1 infected patients in Switzerland [abstract no. TUPDD203]. 17th International AIDS Conference; 2008 Aug 3–8; Mexico City
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: F. Cainelli, School of Medicine, Faculty of Health Sciences, University of Botswana, Gaborone, Botswana; B. Gazzard, St Stephen’s Centre, Chelsea and Westminster Hospital, London, UK; D.T. Jayaweera, Division of Infectious Diseases, University of Miami School of Medicine, Miami, Florida, USA; M. Nelson, HIV Medicine, Chelsea and Westminster Hospital, London, UK; R. Wood, Institute of Infectious Disease and Molecular Medicine, University of Cape Town Faculty of Health Sciences, Cape Town, South Africa.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘raltegravir’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Wolters Kluwer Health ∣ Adis). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search term was ‘raltegravir’. Searches were last updated 20 May 2009.
Selection: Studies in patients with HIV infection who received raltegravir. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Raltegravir, HIV infection, antiretroviral therapy, integrase inhibitor, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Croxtall, J.D., Keam, S.J. Raltegravir. Drugs 69, 1059–1075 (2009). https://doi.org/10.2165/00003495-200969080-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200969080-00007