Abstract
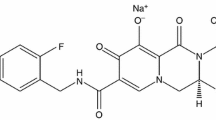
Oral dolutegravir (Tivicay®) is a new-generation HIV-1 integrase strand transfer inhibitor approved as a component of antiretroviral treatment of HIV-1 infection in adolescents and adults. It may be administered once daily, does not require pharmacokinetic boosting, has a high barrier to resistance and is generally active against viral strains resistant to first-generation integrase inhibitors. In clinical trials in treatment-naïve or -experienced patients, dolutegravir was noninferior or superior to raltegravir-, efavirenz-and darunavir/ritonavir-based combinations with regard to viral suppression at week 48, and was generally well tolerated.

Similar content being viewed by others
References
Pandey KK, Grandgenett DP. HIV-1 integrase strand transfer inhibitors: novel insights into their mechanism of action. Retrovirology. 2008;2:11–6.
Isentress (raltegravir): summary of product characteristics. London: European Medicines Agency; 2013.
Vitekta (elvitegravir): summary of product characteristics. London: European Medicines Agency; 2013.
Tivicay (dolutegravir): summary of product characteristics. London: European Medicines Agency; 2014.
Tivicay (dolutegravir) tablets for oral use: US prescribing information. Research Triangle Park: ViiV Healthcare; 2013.
ViiV Healthcare, Shionogi Co Ltd. Tivicay® tablets 50 mg, HIV integrase inhibitor launched in Japan: new treatment option for treatment-naive patients and patients requiring alternative to current treatment regime [media release]. 2014. http://www.shionogi.co.jp/en/company/news/2014/pmrltj0000001zh0-att/e140417.pdf. Accessed 16 June 2015.
Adams JL, Patterson KB, Prince HM, et al. Single and multiple dose pharmacokinetics of dolutegravir in the genital tract of HIV-negative women. Antivir Ther. 2013;18(8):1005–13.
Greener BN, Patterson KB, Prince HMA, et al. Dolutegravir pharmacokinetics in the genital tract and colorectum of HIV-negative men after single and multiple dosing. J Acquir Immune Defic Syndr. 2013;64(1):39–44.
Hightower KE, Wang R, DeAnda F, et al. Dolutegravir (S/GSK1349572) exhibits significantly slower dissociation than raltegravir and elvitegravir from wild-type and integrase inhibitor-resistant HIV-1 integrase-DNA complexes. Antimicrob Agents Chemother. 2011;55(10):4552–9.
Kobayashi M, Yoshinaga T, Seki T, et al. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother. 2011;55(2):813–21.
Underwood MR, Johns BA, Sato A, et al. The activity of the integrase inhibitor dolutegravir against HIV-1 variants isolated from raltegravir-treated adults. J Acquir Immune Defic Syndr. 2012;61(3):297–301.
Canducci F, Ceresola ER, Boeri E, et al. Cross-resistance profile of the novel integrase inhibitor dolutegravir (S/GSK1349572) using clonal viral variants selected in patients failing raltegravir. J Infect Dis. 2011;204(11):1811–5.
Canducci F, Ceresola ER, Saita D, et al. In vitro phenotypes to elvitegravir and dolutegravir in primary macrophages and lymphocytes of clonal recombinant viral variants selected in patients failing raltegravir. J Antimicrob Chemother. 2013;68(11):2525–32.
Huang W, Frantzell A, Whitcomb JM, et al. Impact of raltegravir/elvitegravir selected mutations on dolutegravir cross-resistance [abstract no. 595]. Top Antivir Med. 2014;22(e-1):292–3.
Quashie PK, Mesplède T, Han Y-S, et al. Characterization of the R263K mutation in HIV-1 integrase that confers low-level resistance to the second-generation integrase strand transfer inhibitor dolutegravir. J Virol. 2012;86(5):2696–705.
Mesplède T, Quashie PK, Osman N, et al. Viral fitness cost prevents HIV-1 from evading dolutegravir drug pressure. Retrovirology. 2013;10:22.
Clotet B, Feinberg J, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383(9936):2222–31.
Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–18.
Raffi F, Rachlis A, Stellbrink H-J, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735–43.
van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis. 2012;12(2):111–8.
Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2013;13(11):927–35.
Walmsley S, Berenguer J, Khuong-Josses M-A, et al. Dolutegravir regimen statistically superior to tenofovir/emtricitabine/efavirenz: 96-wk data [abstract no. 543]. Top Antivir Med. 2014;22(e-1):261–2.
Molina JM, Clotet B, Van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomised, open-label, phase 3b study. Lancet HIV. 2015;2(4):e127–36.
Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893):700–8.
Castagna A, Maggiolo F, Penco G, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis. 2014;210(3):354–62.
Eron JJ, Clotet B, Durant J, et al. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING Study. J Infect Dis. 2013;207(5):740–8.
Vavro C, Huang J, Avatapally C, et al. Durable efficacy and limited integrase resistance in subjects receiving dolutegravir after failing a prior INI regimen: week 48 results from VIKING-3 [abstract no. O_10]. Rev Antiviral Ther Infect Dis. 2014;2:13.
Viani R, Alvero C, Fenton T, et al. Safety and efficacy of dolutegravir in HIV treatment-experienced adolescents: 48 week results [abstract no. 906LB]. Top Antivir Med. 2014;22(e-1):474–5.
McCormack PL. Dolutegravir: a review of its use in the management of HIV-1 infection in adolescents and adults. Drugs. 2014;74(11):1241–52.
Acknowledgments
The manuscript was reviewed by: P. Domingo, Infectious Diseases Unit, Hospital Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain; M. A. Wainberg, McGill University AIDS Centre, Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Quebec, Canada; J. van Lunzen, Infectious Diseases Unit, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article was updated from Drugs 2014;74(11):1241–52 [29] by a salaried employee of Adis/Springer and was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from comments received were made by the author on the basis of scientific merit.
Rights and permissions
About this article
Cite this article
McCormack, P.L. Dolutegravir in HIV-1 infection: a guide to its use. Drugs Ther Perspect 31, 259–265 (2015). https://doi.org/10.1007/s40267-015-0226-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-015-0226-9