Summary
Abstract
Doripenem, a parenteral, broad-spectrum antibacterial agent of the carbapenem family, is indicated as empirical therapy in serious bacterial infections in adults. Doripenem is indicated in Japan for use as a single agent in intra-abdominal infections (IAIs), lower respiratory tract infections (including nosocomial pneumonia), complicated urinary tract infections (cUTIs) and a variety of other bacterial infections, such as complicated skin and skin structure infections (cSSSIs), obstetric and gynaecological infections, serious ear, nose and throat infections, sepsis and endocarditis, dental and oral surgical infection, and ophthalmic infection caused by various susceptible strains of Gram-negative, Gram-positive or anaerobic bacteria. Doripenem is indicated in the US for the treatment of complicated IAIs (cIAIs) or cUTIs, including pyelonephritis, caused by susceptible strains of designated pathogens, and in the EU for the treatment of nosocomial pneumonia (including ventilator-associated pneumonia [VAP]), cIAIs or cUTIs.
Doripenem has a broad spectrum of in vitro activity against Gram-positive and Gram-negative bacteria, including extended-spectrum β-lactamase (ESBL)- and AmpC-producing Enterobacteriaceae, and anaerobic pathogens. The drug also has a low propensity to select for resistance and is suitable for the prolonged infusions that may be required to achieve pharmacodynamic/pharmacokinetic targets for bactericidal activity (and therefore efficacy) against pathogens with increased MICs (minimum concentrations required to inhibit the pathogens). Doripenem is no less effective than other antibacterial agents, including meropenem, imipenem/cilastin, piperacillin/tazobactam or levofloxacin in a wide range of serious bacterial infections, such as complicated lower respiratory infections, nosocomial pneumonia (including VAP), cIAIs and cUTIs, and is well tolerated. Thus, doripenem is a valuable addition to the options available for the empirical treatment of serious bacterial infections in hospitalized patients.
Pharmacological Properties
Doripenem demonstrated good in vitro activity against clinically relevant Enterobacteriaceae (Citrobacter spp., Enterobacter spp., Escherichia coli, Klebsiella spp., Morganella morganii, Proteus spp. and Serratia spp.). The minimum concentration inhibiting 90% of strains (MIC90) was generally ≤0.5 mg/L and susceptibility rates were 93–100%. Doripenem was active against ESBL- and AmpC-producing Enterobacteriaceae, with little or no change in MIC90 values compared with non-ESBL- and non-AmpC-producing strains. Doripenem was also active against Haemophilus influenzae, Moraxella catarrhalis and Providencia spp. (MIC90 ≤1.56 mg/L). Activity against non-fermentative Gram-negative pathogens was more limited. Doripenem MIC90 values were 0.2–12.5 mg/L against susceptible isolates of Pseudomonas aeruginosa and 8–64 mg/L against carbapenem- or ceftazidime-resistant isolates. Doripenem activity against Acinetobacter spp. was more limited in Europe and the Americas (MIC90 1–32 mg/L) than in Japan (MIC90 ≤3.13 mg/L).
Doripenem demonstrated good in vitro activity against Gram-positive pathogens, including Staphylococcus aureus (methicillin/oxacillin-susceptible isolates), Streptococcus pneumoniae (including penicillin-, ceftriaxone- or multidrug-resistant strains), S. pyogenes and S. agalactiae (MIC90 ≤1 mg/L; susceptibility rate of 100%), but had limited activity against Enterococcus faecalis (MIC90 4–16 mg/L). Activity against S. epidermidis varied according to geographic region (e.g. for methicillin or oxacillin-susceptible isolates, MIC90 values were 0.03 mg/L in Europe and the Americas and ≤0.031–12.5 mg/L in Japan). Doripenem lacked activity against methicillin or oxacillin-resistant staphylococci and E. faecium.
Doripenem demonstrated in vitro activity against a range of anaerobic pathogens, including B. fragilis, B. thetaiotaomicron and Prevotella spp. (MIC90 0.062–2 mg/L; susceptibility rates of 96–100%). Doripenem activity against Peptostreptococcus spp. varied (MIC90 0.063–6.25 mg/L).
Doripenem has rapid, time-dependent bactericidal activity, a post-antibiotic effect of ≈2 hours against P. aeruginosa and S. aureus at 2 or 4 times the MIC, and no inoculum effect.
Doripenem shows stability against hydrolysis by most β-lactamases, including ESBLs and AmpC β-lactamases, but may be affected by carbapenemases. However, it is likely that in addition to carbapenemases, resistance mechanisms such as reduced permeability or overexpression of multidrug efflux pumps are required for significant carbapenem resistance to emerge. Doripenem appears to have a low potential for selecting resistant strains in vitro.
Doripenem was generally at least as effective as meropenem/cilastin, imipenem/cilastin, biapenem, cefoxatime or cefpirome, and was more effective than ceftazidime, cefepime, ampicillin or piperacillin/tazobactam in various mouse or rat models of infection with clinically relevant Gram-negative, Gram-positive or anaerobic pathogens.
Doripenem did not accumulate at steady state after intravenous administration. Plasma protein binding is low (≈8%) and the drug achieves good penetration into a wide range of tissues, including lung, intra-abdominal, dermal, and head and neck tissue, bone, and peritoneal fluid. Doripenem is mainly eliminated via the kidneys and clinically significant alterations to the pharmacokinetics of the drug are seen in patients with advanced or end-stage renal failure. Doripenem has a short plasma elimination half-life of ≈1 hour.
Mathematical models have estimated the simulated dosage regimen of doripenem that is likely to achieve an optimal bactericidal pharmacodynamic target attainment (unbound drug concentrations that are maintained above MIC for 30–40% of the dose administration interval). In one model, doripenem 250 or 500 mg administered over 30 minutes three times daily was predicted to be the optimal regimen against bacterial isolates with MICs of 1 or 2 mg/L and doripenem 250 mg twice daily was predicted to be optimal against bacterial isolates with MICs ≤0.5 mg/L. In another model, doripenem 500 mg administered over 60 minutes every 8 hours was predicted to be optimal against isolates with MICs ≤2 mg/L, while prolonged (4-hour) infusions of doripenem 500 mg every 8 hours or 1000 mg every 12 hours (MIC 4 mg/L) or 1000 mg every 8 hours (MIC 8 mg/L) were predicted to be optimal against isolates with higher MICs. An increase in dose administration frequency or duration of infusion, rather than dose per administration, was likely to prolong the period over which unbound doripenem concentrations exceeded the MIC.
Therapeutic Efficacy
The efficacy of intravenous doripenem in adult patients with serious bacterial infections has been examined in numerous randomized trials. Doripenem was not inferior to meropenem in Japanese patients with serious lower respiratory tract infection, achieving clinical response rates of >90% in either treatment arm, and bacteriological eradication rates of 86% and 96%, respectively. In two international trials in patients with nosocomial pneumonia (including VAP), doripenem was not inferior to imipenem/cilastin (clinical response rates of 57.8–68.3%) or piperacillin/tazobactam (clinical response rates of 64.1–81.3%) in the clinically evaluable and modified intent-to-treat populations. Bacteriological eradication rates across all treatment arms in these trials were 67.3–84.5%.
Doripenem was not inferior to meropenem in two trials in patients with cIAIs. Clinical cure rates at the test-of-cure assessment were 74.5–85.9% with doripenem and 75.7–85.3% with meropenem. Microbiological cure rates were 84.3% with doripenem and 84.5% with meropenem when results of the two trials were combined.
Doripenem was an effective alternative to meropenem or levofloxacin therapy in patients with cUTIs, evidenced by clinical response rates of 95–96% with doripenem, 89% with meropenem and 90% with levofloxacin. The respective bacteriological response rates were 82–96% for doripenem, 96% for meropenem and 83% for levofloxacin. A Japanese dose-finding trial in patients with cUTIs found that doripenem efficacy did not differ significantly between a 250 or 500 mg dose administered twice daily (clinical response rate of 97% in both treatment groups).
Doripenem has also shown efficacy in small noncomparative Japanese studies in patients with cSSSIs, obstetric and gynaecological infection, sepsis and endocarditis, ear, nose and throat infections, dental and oral surgical infections, and ophthalmic infection (clinical and bacteriological response rates of 80–100%).
Tolerability
Intravenous doripenem was generally well tolerated in adult patients with serious bacterial infections, and most adverse events were mild to moderate in severity. The most commonly reported drug-related adverse events in patients treated with doripenem included headache, nausea and diarrhoea. The most commonly reported laboratory abnormalities included increased levels of ALT and AST. Doripenem has good CNS tolerability, with no drug-related seizures reported in patients participating in clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. Introduction
The carbapenems are β-lactam antibacterial agents with a broad spectrum of activity against a wide range of Gram-positive and Gram-negative aerobic bacteria, and against anaerobic bacteria. However, unlike other β-lactam antibacterials, the carbapenems are stable to nearly all β-lactamases.[1–3] Consequently, in an age of increasing resistance to antibacterial agents, carbapenems are an important therapeutic option, particularly in the treatment of serious infections.[1,2] The most widely available carbapenems are imipenem,[1,2,4] meropenem[5–7] and ertapenem.[8,9] Doripenem (Finibax®, Doribax™),Footnote 1 which has a spectrum of activity similar to that of imipenem and meropenem, is the focus of this review. Other, less widely available carbapenems include biapenem[10] and panipenem.[11]
This article reviews the pharmacological properties of doripenem and its clinical efficacy and tolerability as an intravenous agent in the treatment of adults with lower respiratory tract infections (including nosocomial pneumonia), intra-abdominal infections (IAIs), including complicated IAIs (cIAIs), complicated urinary tract infections (cUTIs) and a variety of other bacterial infections, such as complicated skin and skin structure infections (cSSSIs), obstetric and gynaecological infections, serious ear, nose and throat (ENT) infections, sepsis and endocarditis, dental and oral surgical infection, and ophthalmic infection. Doripenem is approved in a broad range of infections in Japan,[12] for use in cIAIs and cUTIs in the US[13] and EU,[14] and in nosocomial pneumonia (including ventilator-associated pneumonia [VAP]) in the EU.[14]
2. Pharmacodynamic Profile
2.1 Mechanism of Action
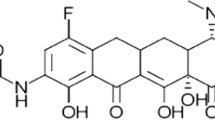
Doripenem (figure 1) is a carbapenem antibacterial agent with a broad spectrum of activity against Gram-positive and Gram-negative bacteria and anaerobes.[3,15] Like other carbapenems, doripenem interferes with cell wall synthesis, inhibiting cell growth and ultimately leading to bacterial cell death.[15]
Doripenem readily penetrates bacterial cell walls, binding with and deactivating specific penicillin-binding proteins (PBPs). PBPs 1a, 1b, 2 and 3 are of primary importance because inhibition of these PBPs leads to cell death.[15] In vitro, doripenem shows strong affinity for PBPs 2, 3 and 4 in Pseudomonas aeruginosa,[12,13,15,16] PBPs 2 and 4 in Escherichia coli[12,13,15,16] and PBP 1 in Staphylococcus aureus[12,16] cell walls, with changes in cell morphology (e.g. formation of spherical cells due to inhibition of PBP 2) apparent 1–2 hours after exposure to the drug.[15,17] Doripenem is highly stable to hydrolysis by all serine β-lactamases, and is relatively stable to human recombinant dehydropeptidase-1 (DHP-1),[16,18,19] unlike imipenem[1] or panipenem.[11]
2.2 In Vitro Antibacterial Activity
The focus of this section is the in vitro activity of doripenem against selected pathogens specified in the US,[13] EU[14] and Japanese[12] manufacturer’s prescribing information (see table I for details). In the US, doripenem is indicated for the treatment of cIAI caused by E. coli, Klebsiella pneumoniae, P. aeruginosa, various Bacteroides spp., S. intermedius, S. constellatus and Peptostreptococcus micros, and for cUTI caused by E. coli, K. pneumoniae, Proteus mirabilis, P. aeruginosa and Acinetobacter baumannii.[13] Doripenem is indicated in adults for the treatment of nosocomial pneumonia (including VAP), cIAI and cUTI in the EU,[14] and in a wider range of indications in Japan, including serious lower respiratory tract infections, IAI, cUTI, cSSSI, obstetric and gynaecological infections, serious ENT infections, sepsis and endocarditis, dental and oral surgical infection, and ophthalmic infection.[12] However, in the EU and Japan, specific organisms are not identified for each of these indications.[12,14] Although the spectrum of doripenem antibacterial activity includes numerous pathogens other than those indicated, the efficacy and tolerability of the drug in infections due to these organisms has not been established in well controlled clinical trials, and the clinical significance of such activity is unclear.[13]
Selected causative pathogens against which intravenous doripenem is indicated for the treatment of complicated intra-abdominal infections or complicated urinary tract infections (US and EU),[13,14] nosocomial pneumonia (including ventilator-associated pneumonia) [EU][14] and various serious infections in Japan[12]
Susceptibility data discussed in this section were collected between 1997 and 2007 in large, worldwide surveillance programmes (including the SENTRY[20] and TRUST[21] programmes)[20–32] and numerous smaller studies (including phase III trials of nosocomial pneumonia,[33,34] cIAIs[34,35] or cUTIs,[34,36] and a study in infected diabetic foot wounds[37]),[33–51] and between 1987 and 2006 in Japanese studies.[16,52–66] Some data are only available as abstracts and posters.[20–31,33–36,38,40,65]
In vitro susceptibility testing was performed according to the Clinical and Laboratory Standards Institute (CLSI) methods for non-Japanese in vitro studies, and Japanese Chemotherapy Society or CLSI methods for Japanese in vitro studies. CLSI breakpoints for doripenem have not yet been established; however, the following breakpoints, which are based on clinical outcomes, are proposed in the US manufacturer’s prescribing information: ≤0.5 mg/L for Enterobacteriaceae, ≤2.0 mg/L for P. aeruginosa, ≤1.0 mg/L for A. baumannii, ≤0.12 mg/L for S. constellatus and S. intermedius, and ≤1.0 mg/L for anaerobes;[13] and in Brown and Traczewski:[39]≤2 mg/L for Enterobacteriaceae, non-fermentative Gram-negative bacilli, Staphylococcus spp. and H. influenzae, and ≤1 mg/L for streptococci. In addition, some studies used a proposed breakpoint of ≤4.0 mg/L for establishing susceptible organisms.[40,44,45,49,51,67] As doripenem-resistant isolates of these organisms have not yet been identified, only susceptibility breakpoints are available in the US.[13,39] Jones et al.[68] have proposed the following possible carbapenem or other β-lactam surrogate agents for doripenem susceptibility testing until doripenem-containing commercial systems are available: oxacillin for methicillin-susceptible S. aureus or coagulase-negative staphylococci; ampicillin for enterococci; meropenem, imipenem or ertapenem for Enterobacteriaceae; meropenem or imipenem for P. aeruginosa and Acinetobacter spp; and ertapenem for H. influenzae and S. pneumoniae. Recent European breakpoints proposed for doripenem are: ≤1 mg/L (susceptible) and >4 mg/L (resistant) against Enterobacteriaceae, Acinetobacter spp. and Pseudomonas spp., and ≤1 mg/L (susceptible) and >1 mg/L (resistant) against Streptococcus spp., S. pneumoniae, Haemophilus spp. and anaerobes. Staphylococcal susceptibility and resistance is inferred from the methicillin breakpoint.[14]
Comparator antibacterial agents shown in tables II–IV were selected because they were the comparator agents in the clinical trials discussed in section 5.
In vitro activity of doripenem (DOR) and other antibacterials against aerobic Gram-negative bacteria. Data were collected between 1997 and 2007 in worldwide surveillance programmes (including the SENTRY[20] and TRUST[21] programmes)[20–27,32] and various smaller studies[24,28,29,31,33,35–41,44,45,48,51,67] (Japanese studies are shown separately). Pathogens tested were clinical isolates, apart from drug-resistant pathogens in six studies.[28–30,41,44,51] Testing was performed according to the Clinical and Laboratory Standards Institute (CLSI) methods. Data shown are minimum concentrations inhibiting 90% of strains (MIC90)
In vitro activity of doripenem (DOR) and other antibacterials against aerobic Gram-negative bacteria collected between 1987 and 2006 in Japan.[16,53–58,61,63,65,66] Testing was performed according to the Clinical and Laboratory Standards Institute (CLSI)[16,54,55,58,61,65] or Japanese Society of Chemotherapy[53,56,57,63] methods. Data shown are minimum concentrations inhibiting 90% of strains (MIC90)
2.2.1 Gram-Negative Bacteria
Doripenem demonstrated good in vitro activity against clinically relevant Enterobacteriaceae, including E. coli, Klebsiella spp., Citrobacter spp., Enterobacter spp., Morganella morganii, Proteus spp. and Serratia spp. (tables II and III). The minimum concentration inhibiting 90% of strains (MIC90) was generally ≤0.5 mg/L (tables II and III) and susceptibility rates, where reported, were 93–100%.[23,26,44,45,51] The exceptions were isolates from two Japanese studies[53,57] (one used isolates from patients with cUTIs[53]) in which MIC90 values were 1.56[57] and 3.13[53] mg/L against E. cloacae, 0.78[53] and 6.25[57] mg/L against P. vulgaris, 0.78 mg/L against P. mirabilis and M. morganii,[53] and 6.25 mg/L against S. marcescens,[53,57] and two US studies of selected clinical isolates[39,41] in which the MIC90 against P. mirabilis was 1 mg/L.
Doripenem showed good activity against extended-spectrum β-lactamase (ESBL)-producing, AmpC-producing (ceftazidime-intermediate/resistant), or fluoroquinolone-resistant Enterobacteriaceae.[20,23,29,32–34,36,41,44,47,51] For example, doripenem MIC90 values did not differ between non-ESBL-producing and ESBL-producing E. coli, K. pneumoniae or P. mirabilis isolates (table II); however, in one Japanese study[59] the minimum inhibitory concentration (MIC) was 32 mg/L against one class B ESBL-producing strain of E. coli. Doripenem also retained good antibacterial activity against both AmpC-producing and non-AmpC-producing strains of Enterobacter spp. and S. marcescens (MIC90 ≤0.5 vs ≤0.12 mg/L; susceptibility rates of 100%).[41,51] In one study,[41] the MIC value was 2 mg/L against AmpC-producing P. mirabilis.
Doripenem activity against carbapenemase-producing Klebsiella or Serratia spp. was limited, with MIC90 values of 8 to >64 mg/L.[47]
Doripenem was active against the respiratory pathogens H. influenzae and Moraxella catarrhalis (MIC90 ≤1.56 mg/L), and against Providencia spp. (MIC90 ≤1.56 mg/L), although the MIC90 for the β-lactamase non-producing, ampicillin-resistant strains of H. influenzae was 2–3.13mg/L (tables II and III).
Doripenem was active against P. aeruginosa isolates across all geographic areas (MIC90 0.2–12.5 mg/L; tables II and III) [where evaluated, 87–100% susceptibility[26,45,51] ], including ceftazidime- or imipenem-susceptible[28] or non-multidrug-resistant[38] isolates (MIC90 of 2–4 mg/L) obtained from hospital inpatients in the US[38] or Europe,[28] and ceftazidime- or imipenem-susceptible or ceftazidime-resistant Japanese isolates (including PER-1-type ESBL-producing P. aeruginosa[52]) [MIC90 ≤4 mg/L].[52,58,63] However, doripenem had poor activity against metallo-β-lactamase-producing or ceftazidime-, imipenem- or multidrug-resistant P. aeruginosa isolates evidenced by MIC90 values of 8–64 mg/L (tables II and III) and, where reported, susceptibility rates of 22–40%.[40,51,67]
Compared with activity against the parent wild-type strain, doripenem activity was increased against P. aeruginosa mutants lacking mexAB-oprM[58,62] (probably due to reduced expression of AmpC β-lactamase[62]), and virtually unchanged against mutant strains of P. aeruginosa (OprD deficiency [reduced porin production] and overexpression of mexAB-oprM or mexEF-oprN [increased efflux pump activity]), with MIC90 values of 1.0 versus 0.5 mg/L.[58] However, the activity of other carbapenems was reduced against strains that had the combination of porin deficiency and increased efflux pump activity (by up to 4-fold for meropenem, up to 8-fold for imipenem and up to 16-fold for biapenem).[58]
Doripenem showed limited activity against Acinetobacter spp. (including A. baumanii) in studies in Europe and the Americas, regardless of whether isolates were susceptible or ceftazidime-intermediate or -resistant (MIC90 1–32 mg/L; table II) [where reported, susceptibility rates 76–78%[26,45,51] ] or carbapenemase- or metallo-β-lactamase-producing (MICs 16–32 mg/L).[47] The MIC90 values for Acinetobacter spp. isolated in Japan were ≤3.13 mg/L (table III).
The antibacterial activity of doripenem against Gram-negative pathogens, including P. aeruginosa and E. coli, was not affected by inoculum size.[16,70]
2.2.2 Gram-Positive Bacteria
Doripenem had good in vitro activity against the Gram-positive pathogens S. aureus or S. haemolyticus (methicillin- or oxacillin-susceptible isolates), Streptococcus pneumoniae (including ceftriaxone-resistant, penicillin-intermediate or -resistant or multidrug-resistant isolates), S. pyogenes and S. agalactiae, with MIC90 values of ≤1 mg/L across all geographic regions (tables IV and V) and, where reported, susceptibility rates of 100%.[25,27,44,45] However, S. epidermidis susceptibility to doripenem varied according to geographic region, with MIC90 values of 0.03 mg/L (methicillin- or oxacillin-susceptible isolates) and 4 mg/L (oxacillin resistant) in Europe and the Americas (table IV) compared with MIC90 values of ≤0.031–12.5mg/L (methicillin susceptible) and 8–50 mg/L (methicillin resistant) in Japan (table V).
In vitro activity of doripenem (DOR) and other antibacterials against aerobic Gram-positive bacteria. Data were collected between 1997 and 2007 in world-wide surveillance programmes[24–27,32] and various smaller studies[30,35–37,39,41,44,45] (Japanese studies are shown separately). Pathogens tested were clinical isolates, apart from drug-resistant pathogens in two studies.[30,41] Testing was performed according to the Clinical and Laboratory Standards Institute (CLSI) methods. Data shown are minimum concentrations inhibiting 90% of strains (MIC90)
In vitro activity of doripenem (DOR) and other antibacterials against aerobic Gram-positive bacteria collected between 1987 and 2006 in Japan.[16,53–55,57,63,65,66] Testing was generally performed according to the Clinical and Laboratory Standards Institute (CLSI)[16,54,55,65,66] or Japanese Society of Chemotherapy[53,57,63] methods. Data shown are minimum concentrations inhibiting 90% of strains (MIC90)
As expected, doripenem did not demonstrate activity against methicillin- or oxacillin-resistant S. aureus (MIC90 8–64 mg/L) [tables IV and V]. Doripenem showed limited activity against Enterococcus faecalis (MIC90 4–16 mg/L) and poor activity against other Enterococci, including E. faecium (MIC90 >32 to >128 mg/L) [table IV and table V].
The antibacterial activity of doripenem against Gram-positive pathogens, including S. aureus, was not affected by inoculum size.[16,70]
2.2.3 Anaerobic Bacteria
Doripenem showed good activity against a range of anaerobic bacteria (tables VI and VII), although regional variations in susceptibility were evident. For instance, doripenem MIC90 values against B. fragilis isolates from Europe and the Americas were 0.25–1 mg/L (susceptibility rates of 96–100%[45,49]), whereas MIC90 values against Japanese isolates were 0.78–2mg/L (table VI and table VII). Likewise, for Peptostreptococcus spp., the doripenem MIC90 was 0.25 mg/L against European and American isolates, and 0.063–6.25mg/L against Japanese isolates (table VI and table VII). Doripenem also showed activity against toxigenic C. difficile clinical isolates (MIC90 2 mg/L); the meropenem MIC90 against these isolates was also 2 mg/L.[42]
In vitro activity of doripenem (DOR) and other antibacterials against anaerobic bacteria. Data were collected in various worldwide studies between 1997 and 2007.[37,43,45,49] Testing was performed according to the Clinical and Laboratory Standards Institute (CLSI) methods. Data shown are minimum concentrations inhibiting 90% of strains (MIC90)
In vitro activity of doripenem (DOR) and other antibacterials against anaerobic bacteria isolated in Japan between 1987 and 2005.[16,54,60,63] Testing was generally performed according to the Clinical and Laboratory Standards Institute (CLSI)[16,54] or Japanese Society of Chemotherapy[60,63] methods. Data shown are minimum concentrations inhibiting 90% of strains (MIC90)
2.2.4 Synergistic Activity
Little antagonistic potential has been observed in in vitro synergy tests with doripenem and amikacin, cotrimoxazole, daptomycin, levofloxacin, linezolid, vancomycin, teicoplanin or ceftobiprole.[13,72–74]
Preliminary data suggest a strong synergistic effect in vitro between the investigational metallo-β-lactamase inhibitor CP 3242 and carbapenems, including doripenem, against some carbapenemase-producing pathogens [reported as an abstract].[75] For instance, MIC values against an IMP-1-producing P. aeruginosa strain, which were 8 mg/L for doripenem and ≥256 mg/L for imipenem or meropenem, were reduced to 1 mg/L for doripenem and 0.5 mg/L for imipenem or meropenem in the presence of 32 g/L of CP 3242 using broth dilution methods.[75] Likewise, in the presence of CP 3242, MIC values against an IMP-2-producing A. baumanii strain were reduced from 16 mg/L to 1 mg/L for doripenem, 128 mg/L to 1 mg/L for imipenem and >256 mg/L to 2 mg/L for meropenem. Synergistic effects between the carbapenems and CP 3242 against VIM-1- or VIM-2-producing P. aeruginosa strains were not as pronounced (MIC values were 0–16 times [VIM-1] and 0–4 times [VIM-2] lower).[75]
2.2.5 Bactericidal Activity and Post-Antibiotic Effect
Doripenem exhibited rapid, time-dependent bactericidal activity against P. aeruginosa (including an efflux pump over-expressing strain),[58,70,76] E. coli[70] and S. aureus.[70] For instance, a 3–4 log10 reduction in the P. aeruginosa bacterial count was observed over 6–8 hours with doripenem at 2–8 × the MIC.[76] For most strains, killing was only slightly more rapid and extensive at 8 × the MIC than at 2 or 4 × the MIC.[76] The mean MBC : MIC ratio against 20 clinical strains of P. aeruginosa was 1.5 for doripenem, 1.25 for meropenem and 1.7 for imipenem, indicating strong bactericidal activity.[58] In contrast, the mean MBC : MIC ratio for ceftazidime was 2.45.[58]
Doripenem also showed bactericidal activity (99.9% kill) against various clinically relevant anaerobes (e.g. B. fragilis, B. thetaiotaomicron and P. intermedia) at 2 × the MIC after 48 hours.[43]
Doripenem demonstrated an in vitro post-antibiotic effect (PAE) of 1.9 hours against S. aureus, 1.8–2 hours against P. aeruginosa, 0.3–0.5 hours against E. coli and 0.3 hours against K. pneumoniae (all assessed at 2 or 4 × the MIC).[70,77]
2.3 Resistance Issues
Doripenem shows stability against hydrolysis by most β-lactamases, including AmpC β-lactamase and ESBLs.[16,32] As discussed in section 2.2.1, there was little change in MIC90 values in comparisons of wild-type and ESBL-producing E. coli or K. pneumoniae isolates, or wild-type and AmpC-producing Enterobacter spp. or S. marcescens.[32,51] However, carbapenemases can affect the activity of doripenem.[78] Among the most potent carbapenemases are the class B metallo-β-lactamases,[78] the production of which is intrinsic in S. maltophilia, thus resulting in resistance to doripenem,[32] but can also be acquired by P. aeruginosa and other Gram-negative species.[78,79] Although carbapenemases are still uncommon, carbapenemase-producing Gram-negative pathogens are being reported with increasing frequency.[78]
Other potential resistance mechanisms that can confer resistance to β-lactam antibacterial drugs include alteration of PBPs leading to poor binding affinity (present in bacteria such as Enterococcus spp. and methicillin- or oxacillin-resistant S. aureus [MRSA]), overexpression of multidrug efflux pumps limiting drug accumulation at target sites (detected in some Gram-negative bacteria, including P. aeruginosa[80–82] and B. fragilis[83,84]) and reduced membrane permeability due to loss of OprD, and thus a reduction in porins, in bacterial cell walls.[46,79,81,82]
In in vitro studies, the loss of OprD in P. aeruginosa isolates increased doripenem, meropenem and imipenem MICs.[46] Increasing carbapenem resistance in P. aeruginosa isolates as a result of the overexpression of efflux pumps (including MexAB-OprM) has been documented;[80] doripenem and meropenem MICs were increased in such mutants, whereas imipenem MICs were not affected.[46,80] Nevertheless, a combination of resistance mechanisms, such as the presence of carbapenemases and reduced permeability, or overexpression of multidrug efflux pumps and reduced permeability, is required for significant carbapenem resistance to emerge.[46,85] For example, detectable doripenem resistance in K. pneumoniae isolates[86] requires the presence of both reduced permeability and the presence of a carbapenemase, and reduced permeability and a functional AmpC β-lactamase are required for doripenem resistance in P. aeruginosa isolates[46] (see also section 2.2). Multiple resistance mechanisms are also required for detectable meropenem resistance in P. aeruginosa isolates; in contrast, OprD loss alone may confer resistance to imipenem.[46]
In vitro studies in which characterized AmpC-inducible strains or wild-type clinical isolates of Enterobacteriaceae or P. aeruginosa were exposed to various antibacterial agents (including doripenem, meropenem or imipenem) at 0.5 or 1 times the MIC, revealed differences between the carbapenems in inducing genes associated with resistance.[87,88] For instance, imipenem induction of AmpC β-lactamases was at least 2-fold higher than that of doripenem or meropenem, whereas doripenem and meropenem had similar induction profiles.[87,88] In one of the studies, imipenem induction of porin gene expression was stronger than that seen with doripenem.[87]
Mucoid strains of P. aeruginosa (as opposed to flagellar strains) produce large quantities of exopolysaccharide alginate, which is associated with the evasion of phagocytosis at the site of infection, protection against reactive oxygen intermediates and reduced susceptibility to antibacterial agents.[89] Various studies have suggested that exposure of P. aeruginosa isolates to subinhibitory concentrations of β-lactam antibacterials may increase alginate production (and therefore reducing antibacterial efficacy) by upregulating alginate-producing gene expression.[90] In planktonic cultures of wild-type P. aeruginosa exposed to imipenem, doripenem or meropenem at concentrations 1 times the MIC, alginate biosynthetic operon expression and synthesis was generally unaffected by doripenem or meropenem, whereas with imipenem, alginate biosynthetic operon expression and synthesis was upregulated (p < 0.01 for imipenem vs doripenem) and flagellar gene expression was reduced.[91] Of interest, the response to imipenem was considered to be similar to the phenotype of mucoid strains of P. aeruginosa that are associated with increased mortality in cystic fibrosis patients.[91]
Doripenem generally appears to have a low potential for selecting resistant strains in vitro.[12,13,46,85,92] A study comparing the potencies of doripenem, meropenem and imipenem in preventing the emergence of carbapenem-resistant mutants of P. aeruginosa, found that mutants with decreased or no expression of the outer membrane protein OprD were predominantly selected; relative potencies were doripenem > imipenem > meropenem.[85] Serial passaging studies showed that the combination of doripenem plus an aminoglycoside (gentamicin) was more potent than doripenem alone in preventing selection of resistant P. aeruginosa strains during the first three passages, although MIC values increased 2- to 4-fold during the seven-passage study.[92] Although potential exists for cross-resistance to carbapenem-resistant isolates to develop (decreased susceptibility of S. aureus, E. coli and P. aeruginosa to doripenem, meropenem or imipenem with cross-resistance was seen in a serial passaging study [14 passages[16] ]), some carbapenem-resistant pathogens may be susceptible to doripenem.[12,13]
2.4 In Vivo Antibacterial Activity
In murine models of immunocompetent or neutropenic Gram-negative systemic, respiratory, UTI or skin infection,[52,58,63,70,93–98] or Gram-positive systemic or lung infection,[63,70,95] and rat models of penicillin-resistant pneumococcal meningitis, S. aureus endocarditis or polymicrobial intrauterine infection (E. coli and B. fragilis pyometra),[54] doripenem was generally at least as effective as imipenem/cilastin, meropenem/cilastin, biapenem, cefotaxime or cefpirome, and more effective than ceftazidime, cefepime, ampicillin or piperacillin/tazobactam.[52,54,58,63,70,93–98]
Gram-negative organisms studied included K. pneumoniae, P. vulgaris, H. influenzae (including β-lactamase-producing strains), P. aeruginosa (including a PER-1-type ESBL-producing strain and ceftazidime-, imipenem- or multidrug-resistant strains), A. calcoaceticus, wild-type or β-lactamase-producing strains of E. coli, and ceftazidime-resistant strains of E. cloacae and S. marcescens. Gram-positive organisms studied included S. aureus, S. pyogenes, S. pneumoniae (including penicillin-resistant strains) and E. faecalis. Antibacterial activity was assessed using the dose of drug resulting in the survival of 50% of animals[52,58,63,70,93–95,97] or skin lesion volume and/or bacterial cell count in fluid.[54,63,95,98]
The effective bacterial regrowth time in a murine neutropenic thigh infection model was 7.8 hours against S. aureus and 5 hours against K. pneumoniae after administration of doripenem 50 mg/kg and 8 hours against P. aeruginosa after a 100 mg/kg dose.[77]
In a neutropenic mouse P. aeruginosa lung infection model, the doripenem PAE after a 3 mg/kg dose was 6.12 hours, compared with 5.9 and 3.49 hours for meropenem/cilastin or imipenem/cilastin 3 mg/kg and 0.35 hours for ceftazidime 10 mg/kg.[58]
2.5 Other Effects
Doripenem showed no convulsive activity in in vivo studies in rats, mice or dogs, nor did it have an effect on the anticonvulsant activity of valproic acid in a rat pentylenetetrazol- or bicuculine-induced convulsive model.[99]
3. Pharmacokinetic Properties
The pharmacokinetics of intravenous doripenem after single-[100–104] or multiple-[102,105] dose administration has been assessed in healthy US[105] or Japanese adult volunteers,[102,103] in elderly Japanese[12,101] or US[13] volunteers, and in US[100] or Japanese[12,104] individuals with renal impairment. Tissue penetration of doripenem after intravenous administration has been assessed in a number of studies in Japanese patients.[64,106–112] Drug interaction data for doripenem and valproic acid are from animal studies[113] and case reports.[114] Data from the US,[13] EU[14] and Japanese[12] manufacturer’s prescribing information are supplemented by results from individual pharmacokinetic studies[64,100–112] or analyses,[115] and the manufacturer.[116] Some data[100,105,115] are only available as abstracts and posters.
3.1 Distribution
The pharmacokinetics of doripenem are linear over a dose range of 125–1000 mg, infused over 30 or 60 minutes either as a single dose[102,115] or as a 500 or 1000 mg dose administered two or three times daily for 1–7 days,[102,105] or over 4 hours[14] in both Japanese[102] and non-Japanese[14,105,115] studies, with no evidence of accumulation. Plasma doripenem concentrations are best described by a two-compartment model, according to a population pharmacokinetic model that used data from healthy adult volunteers (n = 24), individuals with renal impairment (n = 20) and patients with cUTIs (n = 107).[115] Mean peak plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC) values after a single dose of doripenem 250 or 500 mg administered as a 1-hour infusion in healthy non-Japanese or Japanese adults are shown in table VIII.
After prolonged (4-hour infusion) intravenous administration of doripenem 500 mg or 1 g in healthy volunteers, mean Cmax values were ≈8 and ≈17 μg/mL, and mean AUC values from time zero to infinity were ≈34 and ≈68 μg ⋅ h/mL.[14]
Plasma protein binding of doripenem is low (≈8%) and is independent of plasma drug concentrations.[13,14] Doripenem distributes into a wide variety of tissues, with a median volume of distribution (Vd) at steady state of 16.8 L, consistent with distribution into the extracellular space.[13] Doripenem penetrates tissues well, and achieves drug concentrations at or above those required to inhibit most susceptible bacteria, although the clinical significance of this finding in retroperitoneal fluid and peritoneal exudate is not established.[13] After a single intravenous dose of 250 or 500 mg, doripenem concentrations were detected in most tissues and/or fluids, including retroperitoneal fluid, peritoneal exudate, gallbladder tissue, bile, urine, dermal tissues, synovial fluid, bone, sputum, prostatic tissue, gynaecological tissues, middle ear mucosa and middle ear secretions, palatine tonsil tissue, aqueous humour, gingival tissue and various cysts.[12–14,64,106–112] Animal studies have shown that after intravenous administration, doripenem is also distributed extensively in the kidney, liver, lung, heart and spleen.[117]
3.2 Metabolism and Elimination
Doripenem is metabolized via DHP-1 to a microbiologically inactive metabolite. Most of a dose of doripenem is not metabolized, as the drug is relatively stable to DHP-1.[13,14] In a study of prolonged-infusion regimens, the mean plasma doripenem metabolite : doripenem AUC ratio was 13% after a single dose of doripenem 500 mg administered over 4 hours and 18% after a single dose of doripenem 1000 mg administered over 4 hours.[116] In vitro studies have shown that doripenem does not undergo metabolism by, and is therefore not a substrate for, hepatic cytochrome P450 (CYP) isoenzymes. During a period of 1 week after administration of a single 500 mg dose of radiolabelled doripenem in healthy volunteers, <1% of the total radioactivity was recovered in faeces.[13]
Doripenem is primarily excreted as the unchanged drug via the kidneys;[12,14] the high renal clearance rate (table VIII) and the significant reduction in doripenem elimination when the drug coadministered with probenecid (section 3.4), suggest that doripenem undergoes both active tubular secretion and glomerular filtration. Urinary elimination is not dose dependent,[12] and 85–90% of a dose is excreted (70–75% as the unchanged drug) within 24–48 hours of administration.[12,13]
3.3 Special Patient Populations
The pharmacokinetics of intravenous doripenem are not affected by age, ethnicity or gender.[12–14] The effect of hepatic impairment on the pharmacokinetics of doripenem has not been studied, but is thought to be of no clinical consequence, because the drug does not undergo hepatic metabolism.[13,14] The pharmacokinetics of doripenem in patients with severe infections or sepsis are not available.
3.3.1 In Renal Impairment
As a result of prolongation of the doripenem terminal elimination half-life (t½β) in patients with renal impairment,[12,100,104] systemic exposure to doripenem is significantly increased in these patients.[12–14,104] In Japanese individuals, mean AUC values after intravenous administration of a single 250 mg dose of doripenem, t½β values in patients with mild (creatinine clearance [CLCR] 50 to <70 mL/min [3.0–4.2 L/h]), moderate (CLCR 30 to <50 mL/min [1.8 to <3.0 L/h]) or severe (CLCR <30 mL/min [<1.8 L/h]) renal impairment were increased 2.2- to 4.0-fold compared with that in healthy volunteers (1.98, 2.16 and 3.56 vs 0.9 hours) and AUC values were increased 2.0- to 3.2-fold (40.55, 48.21 and 64.31 vs 20.26 μg ⋅ h/mL).[104]
Likewise, in non-Japanese individuals, mean AUC values after intravenous administration of a single 500 mg dose of doripenem were increased 1.6-fold in patients with mild renal impairment (CLCR 50–79 mL/min [3.0–4.74 L/h]), 2.8-fold in those with moderate renal impairment (CLCR 31–50 mL/min [1.86–3.0 L/h]) and 5.1-fold in those with severe renal impairment (CLCR ≤30 mL/min [≤1.8 L/h]) compared with aged-matched individuals with normal renal function.[13,14] Dosage adjustments are required in patients with moderate or severe renal impairment (see section 7).[12–14]
In patients with end-stage renal disease undergoing haemodialysis (doripenem was administered post-dialysis), the mean doripenem AUC was increased 7.8-fold compared with individuals with normal renal function; 52% of a dose of doripenem was recovered in dialysate (46% as the unchanged drug and 6% as the metabolite[116]) after a 4-hour haemodialysis session.[13,116] Insufficient data are available for dosage adjustment recommendations in this group of patients.[13]
3.4 Potential Drug Interactions
As doripenem is not metabolized via CYP isoenzymes (section 3.2), it is not expected to inhibit clearance of drugs metabolized via these pathways or to induce these isoenzymes in a clinically relevant manner.[13,14]
When administered concomitantly with probenecid, doripenem elimination decreased, evidenced by a significantly prolonged t½β (p = 0.0002 vs doripenem alone) and significant reductions in systemic and renal clearance, and cumulative urinary excretion of doripenem (all p < 0.01).[103] As a consequence, systemic exposure to doripenem (Cmax and AUC) was significantly increased (both p < 0.01).[103] The concomitant administration of doripenem and probenecid is not recommended.[12–14]
When carbapenems are administered concurrently with valproic acid, serum valproic acid concentrations may be reduced to subtherapeutic levels with subsequent loss of seizure control.[12–14] Consequently, coadministration of doripenem and valproic acid is contraindicated in Japan,[12] and requires frequent monitoring of serum valproic acid concentrations in the US[13] and EU.[14] The mechanism of interaction is not understood, but results of animal and in vitro studies suggest that carbapenems may inhibit valproic acid glucuronide hydrolysis.[113,114]
4. Pharmacodynamic/Pharmacokinetic Relationship
Animal models have shown that for time-dependent antibacterials, such as the carbapenems, the pharmacodynamic/pharmacokinetic parameter that best correlates with bactericidal activity (and therefore efficacy) is the proportion of the dose administration interval that unbound drug concentrations are maintained above the MIC (%T > MICfree).[118–120] A T > MICfree of 30–40% is thought to be sufficient for carbapenems,[118] including doripenem.[119,120] Murine neutropenic thigh infection models showed that achieving a mean T > MICfree of 21–43% produced a 1 or 2 log reduction in bacterial count for S. pneumoniae, S. aureus or Gram-negative bacilli (including ESBL-producing Enterobacteriaceae).[120,121]
A Monte Carlo simulation[120] used these pharmacodynanamic/pharmacokinetic targets and a doripenem population pharmacokinetic model (based on pharmacokinetic data from 24 healthy volunteers receiving a single intravenous 500 mg dose of doripenem [data are shown in table VIII]) as inputs to identify potential doripenem dose administration regimens for phase II and phase III clinical studies.[119] A regimen of doripenem 500 mg administered over 60 minutes every 8 hours was predicted to be effective against bacterial isolates with MICs ≤2 mg/L, based on a 99–100% probability of achieving a T > MICfree of 35% for doripenem MIC values of 1 or 2 mg/L.[119] For pathogens with an MIC of >2 mg/L, prolonged (4-hour) infusions of doripenem 500 mg every 8 hours or 1000 mg every 12 hours (MIC 4 mg/L) or doripenem 1000 mg every 8 hours (MIC 8 mg/L) was predicted to be effective, based on a 100% probability of achieving a T > MICfree of 35%.[119] Prolonging the infusion duration increased the period over which doripenem concentrations exceeded MIC.[119] Estimates generated from a simulation predicting pharmacodynanamic/pharmacokinetic target attainment in individuals with normal or impaired renal function, using the same pharmacodynanamic/pharmacokinetic data were consistent with these results.[122]
In another murine neutropenic thigh infection model, a simulated regimen of doripenem 250 mg twice daily infused over 30 minutes was bactericidal for E. coli, S. aureus and P. aeruginosa clinical isolates with MICs ≤0.5 mg/L.[123] This dosage regimen achieved a T > MICfree of >35%, and bactericidal activity did not increase significantly when the T > MICfree increased to >40% (simulated treatment regimens of doripenem 500 mg twice daily or 250 mg three times daily infused over 30 minutes). Against P. aeruginosa strains with an MIC of 1 mg/L, simulated regimens of doripenem 250 mg three times daily or 500 mg twice daily administered over 30 minutes were required to achieve an adequate T > MICfree (33–42%).
However, against P. aeruginosa strains with an MIC of ≥2 mg/L, more frequent administration was necessary to achieve an adequate T > MICfree value.[123] For instance, a simulated regimen of doripenem 250 or 500 mg three times daily administered over 30 minutes achieved a T > MICfree of 31–38% against strains with an MIC of 2 mg/L, whereas a simulated doripenem 500 mg twice-daily regimen only achieved a T > MICfree of 25%. Against strains with an MIC of 3 mg/L, a simulated regimen of doripenem 500 mg three times daily administered over 30 minutes achieved a T > MICfree of 27%.[123] An increase in dose administration frequency, rather than dose per administration was associated with a more prolonged T > MICfree value and greater bactericidal activity.[123]
A murine neutropenic thigh model of P. aeruginosa infection was used to simulate the T > MICfree value for a regimen of intravenous doripenem 500 mg infused over 1 or 4 hours every 8 hours.[124] The simulated 1-hour infusion regimen was bactericidal for P. aeruginosa isolates with an MIC ≤2 mg/L, but had variable kill for isolates with MICs of 4–8 mg/L. Bactericidal activity was maximal once T > MICfree was ≥40% and bacteriostatic activity occurred when T > MICfree was ≈20–30%. The simulated 4-hour infusion regimen was bactericidal for all P. aeruginosa isolates with an MIC ≤2 mg/L and for two of four isolates with MICs of 4 mg/L.[124] The option to administer doripenem as an extended 4-hour infusion in patients with nosocomial pneumonia (including VAP) in the EU prescribing information (section 7) is based on these results.[14]
Although doripenem Cmax values in peritoneal exudate were half those in serum after a single 500 mg 30-minute intravenous fusion in ten patients undergoing abdominal surgery (25 vs 47 mg/L), time to Cmax values in peritoneal exudate were increased by 40% (0.7 vs 0.5 hours).[106] The mean T > MICfree in peritoneal fluid was 78% for an MIC of 1 mg/L and 42% for an MIC of 4 mg/L, confirming that doripenem 500 mg every 8 hours was an adequate treatment regimen to achieve bactericidal activity against common pathogens associated with intra-abdominal infections[106] (e.g. E. coli [MIC90 ≤0.015–0.1 mg/L; section 2.2.1], Streptococcus spp. [MIC90 ≤0.004–1 mg/L; section 2.2.2] and B. fragilis [MIC90 0.25–2; section 2.2.3]).
A limitation of the Monte Carlo and other simulations discussed in this section is that the pharmacokinetic parameters were based on data from healthy volunteers and may not apply to patient populations who have pharmacokinetics that differ significantly from those studied.[119,124] For instance, patients with severe sepsis or septic shock have pharmacokinetic profiles that differ markedly from healthy volunteers or less critically ill patients, because of aggressive fluid resuscitation, the loss of endothelial barrier function (capillary leak syndrome), renal or hepatic impairment and decreased tissue perfusion resulting from impaired cardiocirculatory function.[125]
5. Therapeutic Efficacy
The therapeutic efficacy of doripenem in the treatment of adult patients (mean age 50–59 years) with lower respiratory tract infections (including nosocomial pneumonia),[126–130] cIAIs[131–133] or cUTIs (including complicated or uncomplicated pyelonephritis)[134,135] has been evaluated in eight randomized, multicentre, phase II or III clinical studies. Several smaller, noncomparative studies in Japanese patients with lower respiratory tract infections,[136] IAIs,[107,136,137] cUTIs,[137–139] skin and skin structure infection,[112] obstetric and gynaecological infection,[108] sepsis and endocarditis,[140] ENT infection,[109,136] dental and oral surgery,[110] and ophthalmic infection[111] are also available. Three non-Japanese studies[126,129,131] are fully published, and a further three[130,132,134] are only available as abstracts and posters. All Japanese studies[107–112,127,128,136–140] are fully published.
Primary efficacy endpoints in the randomized trials related to clinical and/or microbiological response rate, generally assessed at the test-of-cure visit.[126–129,131,132,134,135] Seven of the randomized studies[126,127,129,131,132,134,135] determined the noninferiority of doripenem versus active comparators; however, only six studies specified the criterion for noninferiority: a lower limit of the two-sided 95% confidence interval for the between-group difference in clinical[127,131,132,135] or microbiological[134] cure rate (doripenem minus active comparator) of greater than or equal to −10%,[127,134,135]−15%[131,132] or −20%.[126,129]
The Japanese studies generally used doripenem dosages of 250 mg twice[107–112,127,135–139] or three times[107–111,128,136,137] daily, or 500 mg twice[107–112,128,135–141] or three times[140,141] daily infused over 30–60 minutes. The other clinical studies with doripenem utilized a dosage regimen of 500 mg every 8 hours (infused over 60 minutes),[126,131,132,134] with the exception of one study in patients with nosocomial pneumonia in which doripenem 500 mg every 8 hours was infused over 4 hours.[129]
5.1 Serious Lower Respiratory Tract Infections
The efficacy of intravenous doripenem in patients with serious lower respiratory tract infections has been investigated in four randomized, multicentre trials:[126–129] a double-blind, Japanese, dose-finding study of doripenem 250 or 500 mg twice daily,[128] a double-blind, Japanese, noninferiority comparison of doripenem 250 mg or meropenem 500 mg twice daily[127] (both in patients with chronic respiratory disease and concurrent, acute bacterial respiratory infection[127,128]) and two nonblind, international, noninferiority trials comparisons of doripenem versus imipenem/cilastin or piperacillin/tazobactam in patients with nosocomial pneumonia[126,129] (one study only enrolled patients with VAP[129]). Results of three small, noncomparative, Japanese studies of doripenem 250 mg two or three times daily, or 500 mg twice daily (in chronic respiratory disease or community-acquired pneumonia [CAP])[136,137] and doripenem 500 mg two or three times daily, 1 g twice daily or 500 mg twice daily titrated down to 250 mg twice daily based on symptom improvement and age (in nosocomial pneumonia)[141] are also discussed briefly. Treatment duration varied between 3 and 15 days.[126–129,136,137,141]
In the two nonblind international trials (section 5.1.1), doripenem 500 mg three times daily[126,129] (the trial in patients with VAP used a 4-hour rather than the standard 1-hour infusion[129]) was compared with imipenem/cilastin 500 mg four times daily or 1000 mg three times daily in one study,[129] or piperacillin/tazobactam 4.5 g four times daily in the other study.[126] A pooled analysis of these data (available as an abstract)[130] and an assessment of hospital resource utilization in the comparison between doripenem and imipenem/cilastin[142] have been performed. Randomization in both trials was stratified by geographic region,[126,129] disease severity (Acute Physiology and Chronic Health Evaluation [APACHE] II score ≤15 or >15),[126,129] the presence or absence of VAP[126] and/or the duration of mechanical ventilation (<5 or ≥5 days).[129] Both trials recommended coadministration of an aminoglycoside[126,129] (amikacin[129]) [in suspected P. aeruginosa infection] or vancomycin[126,129] (in suspected MRSA infection) where required. Patients in the study that compared doripenem with piperacillin/tazobactam[126] could be switched to oral levofloxacin 750 mg once daily after 3 days of intravenous therapy (9 doses of doripenem and 12 doses of piperacillin/tazobactam).
Patients in the VAP study were required to meet the clinical and radiological criteria for VAP, have a Clinical Pulmonary Infection Score of ≥5 and either have been mechanically ventilated for >24 hours or weaned from mechanical ventilation within the previous 24 hours.[129] At baseline in the clinically evaluable population (per-protocol evaluable; n = 248[129]), 48% of patients in the VAP study had APACHE II scores ≤15, 10% of patients were bacteraemic, 39% had early onset VAP (<5 days) and 19% had renal impairment (CLCR <4.8 L/h [<80 mL/min]).[129] In the other study (n = 253 clinically evaluable patients),[126] 75% of patients had APACHE II scores ≤15, 10% of patients were bacteraemic, 22% had early onset VAP (<5 days) and 44%[126] had renal impairment (CLCR <4.8 L/h [<80 mL/min]).
In the randomized, double-blind, Japanese trials, clinical efficacy statistical analyses were primarily conducted in the per-protocol population, supported by results in the modified intent-to-treat (mITT) population.[127,128] In the noninferiority trial, only patients with moderate, non-MRSA lower respiratory tract infection who complied with the treatment protocol were eligible for inclusion in the clinical efficacy per-protocol analysis (n = 96 doripenem and 97 meropenem recipients).[127] Chronic respiratory disease included chronic bronchitis, bronchiectasis, asthma, emphysema, pulmonary fibrosis or previous pulmonary tuberculosis.[127] Diagnoses included bacterial pneumonia (n = 131) or chronic respiratory disease with concurrent, acute respiratory infection (n = 62). Underlying disease or complications were present in 85% of patients and 38% had already been treated with another antibacterial agent.[127]
In all Japanese trials, clinical efficacy refers to percentages of patients with ‘excellent’ or ‘good’ clinical responses,[128,136,137]‘clinical cure’[141] or ‘effective’ treatment.[127,128]
Co-primary efficacy endpoints in the nonblind, international trials were clinical cure rates at the test-of-cure (TOC) visit (7–14 days after the cessation of therapy in the VAP study[129] or 6–20 days after the cessation of therapy in the comparison with piperacillin/tazobactam[126]) in the clinically evaluable[126,129] and clinical mITT (patients who met the definition of the respiratory tract infection being studied and received any amount of the study drug [n = 501;[129] n = 429[126] ]) populations.
In all trials,[126–129,136,137,141] bacteriological response, which included eradication or presumed eradication of pathogens isolated at baseline, was assessed in the microbiologically evaluable population (defined in one trial[129] as the clinically evaluable population who had at least one baseline pathogen that was susceptible or of intermediate susceptibility to the study drug).
The most frequent causative pathogens isolated at baseline were S. pneumoniae,[127–129,136] other Streptococcus spp.,[129] H. influenzae,[127–129] P. aeruginosa,[126,129,136] S. aureus,[126,129] K. pneumoniae[126,129] and E. cloacae.[126,129] In the randomized trials of nosocomial pneumonia,[129] 13% of P. aeruginosa isolates were resistant to imipenem/cilastin,[129] while 27% of P. aeruginosa and 44% of K. pneumoniae isolates were resistant to piperacillin/tazobactam.[126] Few P. aeruginosa isolates (none in the comparison with imipenem/cilastin[129] and 8% in the comparison with piperacillin/tazobactam[126]) and no K. pneumoniae isolates[126] were resistant to doripenem. Most (≥64%) S. aureus isolates were methicillin susceptible.[126,129]
A dose-finding trial found that doripenem 250 mg twice daily was the optimal dosage in Japanese patients with moderately severe chronic respiratory infection.[128] Clinical efficacy rates with doripenem 250 mg twice daily or 500 mg twice daily at the end-of-treatment (EOT) visit were 100% and 88% in the per-protocol population (n = 36 and 34) and 89% and 84% in the mITT population (n = 44 and 38); no significant between-group differences was evident. Likewise, there was no significant difference in bacterial eradication rates between the two treatment groups (94.1% vs 89.5%; n = 17 and 19 evaluable patients).[128]
Doripenem was not inferior to meropenem in the treatment of lower respiratory infections, according to results of a Japanese trial.[127] In patients with CAP or chronic respiratory disease with acute infections, doripenem was noninferior to meropenem, with a clinical efficacy rate ≥90% (primary efficacy endpoint; table IX). Results in the per-protocol population were supported by mITT analyses (full analysis set), in that clinical efficacy was evident in 88.9% of doripenem (n = 108) and 86.8% of meropenem (n = 106) recipients (between-group difference 2.1%; 95% CI −6.7, 10.9).[127] Bacteriological eradication was seen in 86.0% of doripenem and 95.8% of meropenem recipients. At the post-therapy follow-up visit (n = 99), 11.4% of doripenem and 7.3% of meropenem recipients had evidence of recurrent infection or re-infection.[127] When analysed by diagnosis (CAP or chronic respiratory tract infection), the presence or absence of underlying disease or complicating factors, or previous antibacterial therapy, clinical efficacy rates were ≥89% and did not differ between doripenem or meropenem.[127]
Efficacy of intravenous doripenem (DOR) in serious bacterial lower respiratory tract infections. Results of randomized trials that compared DOR with imipenem/cilastin (IPM),[129] meropenem (MEM)[127] or piperacillin/tazobactam (TZP)[126] in patients (pts) with nosocomial pneumonia (including one trial[129] in pts with ventilator-associated pneumonia [VAP][126,129] or other serious lower respiratory tract infections.[127] Study drugs were administered intravenously
In patients with chronic respiratory tract infection or CAP and other pulmonary infection (n = 102[136] and 41[137]) that were included in two noncomparative trials of mixed infections, doripenem 125 mg twice daily, 250 mg two or three times daily or 500 mg twice daily achieved clinical response rates of 85–95% at treatment end and bacteriological eradication rates of 86–100%.[136,137]
5.1.1 Nosocomial Pneumonia
Doripenem was not inferior to imipenem/cilastin[129] or piperacillin/tazobactam[126] in patients with nosocomial pneumonia, including those with VAP. Clinical cure rates with doripenem at the TOC visit were noninferior to those with either comparator in both the clinically evaluable (table IX) and clinical mITT populations. In the latter analyses, a clinical cure was seen in 59.0% of doripenem and 57.8% of imipenem/cilastin recipients with VAP (between-group difference 1.2%; 95% CI −7.9, 10.3),[129] and 69.5% of doripenem and 64.1% of piperacillin/tazobactam recipients with nosocomial pneumonia (between-group difference 5.4%; 95% CI −4.1, 14.8).[126] Combined clinical cure rates in the microbiologically evaluable population at the TOC visit were 74.5% with doripenem (n = 200) and 70.5% with the comparators (imipenem/cilastin or piperacillin/tazobactam) [n = 193].[130]
Patients receiving doripenem (n = 249) experienced a significantly shorter median hospital length of stay (22 vs 27 days; p = 0.01) and duration of mechanical ventilation (7 vs 10 days; p = 0.03) than imipenem/cilastin recipients (n = 250), although length of stay in the intensive care unit with either treatment did not differ (median of 12 vs 13 days) [clinical mITT analysis].[142]
Subgroup analyses showed that doripenem maintained good efficacy in higher risk patient subgroups. For instance, clinical cure rates were ≥57% with doripenem,[126,129] imipenem/cilastin[129] or piperacillin/tazobactam[126] in patients with nosocomial pneumonia (including VAP) who were elderly (aged ≥65 years)[126,129] or had APACHE II scores >15[126] or >20.[129]
Microbiological cure rates with doripenem, imipenem/cilastin or piperacillin/tazobactam in the microbiologically evaluable population were ≥67% (table IX).[126,129] Pooled results in the microbiologically evaluable population showed that the microbiological cure rate was 78.0% (156 of 200 patients) with doripenem and 73.1% (141 of 193 patients) with imipenem/cilastin or piperacillin/tazobactam and per-pathogen eradication rates with doripenem were ≥74%.[130] Microbiological cure rates by selected bacterial pathogen from the two clinical trials[126,129] are shown in figure 2. In patients with P. aeruginosa, K. pneumoniae or E. coli infections at baseline in the VAP study, microbiological cure rates were numerically higher with doripenem than imipenem/cilastin (figure 2); however, the number of isolates in each comparison were small and between-treatment differences were not statistically significant.[129]
Efficacy of intravenous doripenem (DOR) in patients with ventilator-associated pneumonia (VAP) or nosocomial pneumonia. Microbiological response rates by pathogen in the microbiologically evaluable population in two randomized, open-label, multicentre clinical trials comparing DOR with (a) piperacillin/tazobactam (TZP)[126] and (b) imipenem/cilastin (IPM).[129] Adult patients received DOR 500 mg every 8 hours[126,129] (infused over 4 hours, rather than the standard 1 hour, in the trial in patients with VAP[129]), TZP 4.5 g every 6 hours[126] or IMP 500 mg every 6 hours, or 1000 mg every 8 hours[129] for 7–14 days. Where reported,[129] eligible patients had a measurable outcome at the test-of-cure visit and at least one baseline pathogen susceptible (or of intermediate susceptibility) to the study drug. Microbiological cure was usually defined as eradication or presumed eradication.[129] The number of patients in whom the individual pathogens were isolated at baseline are shown above each bar. MSSA = methicillin-susceptible Staphylococcus aureus.
A clinical cure was achieved in 13 of 14 evaluable patients with nosocomial pneumonia who received doripenem 1–2 g/day as divided doses two or three times daily for 8–13 days in a small Japanese trial.[141]
5.2 Complicated Intra-Abdominal Infection
The efficacy of intravenous doripenem 500 mg three times daily was compared with that of intravenous meropenem 1000 mg three times daily in two randomized, double-blind, double-dummy, noninferiority trials in patients with cIAIs requiring parenteral antibacterial therapy (one fully published[131] and the other[132] reported as an abstract and poster). An analysis of the combined results of these two studies (reported as an abstract and poster)[133] is also available. The duration of study therapy was a minimum of 5 days and a maximum of 14 days; however, patients could be switched to oral amoxicillin/clavulanic acid after at least nine doses of doripenem or meropenem.[132] Randomization was stratified by geographic region, and within each region, by primary site of infection (localized appendicitis vs other sites of IAI) and by severity of illness (APACHE II score ≤10 or >10).[131–133] E. coli was the most frequently isolated pathogen at baseline.[131,132]
The co-primary efficacy endpoints were clinical response at the TOC visit 21–60 days after completing treatment with the study drug (intravenous doripenem or meropenem alone or doripenem or meropenem followed by oral amoxicillin/clavulanic acid therapy) in the microbiologically evaluable population and clinical response in the the microbiological mITT (mMITT) population up to 60 days after the last dose of the study drug.[131,132]
Doripenem was not inferior to meropenem in the treatment of cIAI.[131,132] Clinical cure rates at the TOC assessment were ≥75%, and outcomes with doripenem were noninferior to those with meropenem in both the microbiologically evaluable (table X) and the mMITT (co-primary endpoints) patient populations (77.9% vs 78.9%, between-group difference −1.0% [95% CI −9.7, 7.7] in one study;[131] and 74.5% vs 75.7%, between-group difference −1.2% [95% CI −10.3, 8.0] in the other[132]). The mMITT patient population included 195[131] and 200[132] doripenem recipients and 190[131] and 185[132] meropenem recipients. Doripenem showed good bacterial eradication rates for organisms associated with cIAIs, including Enterobacteriaceae, P. aeruginosa and viridans Streptococci group;[131,132] for instance, eradication rates with doripenem or meropenem for E. coli were 87.5% and 84.0% (between-group difference 3.5%; 95% CI not reported) in one study[131] and 87.5% and 84.8% (between-group difference 2.7; 95% CI −7.6, 13.0) in the other.[132]
Efficacy of intravenous doripenem (DOR) in complicated intra-abdominal infection. Results of two randomized, double-blind, double-dummy, multicentre trials that compared DOR with meropenem (MEM).[131,132,135] Planned treatment duration was 5–14 days. DOR and MEM were administered intravenously and patients (pts) could be switched to oral amoxicillin/clavulanic acid after at least nine doses of DOR or MEM. One study reported as an abstract and poster[132]
An analysis of combined results from these two trials confirmed the noninferiority of doripenem to meropenem in cIAI.[133] Clinical cure rates in the microbiologically evaluable population at EOT was 84.6% for doripenem (n = 325) and 84.1% for meropenem (n = 309) [between-group difference 0.5%; 95% CI −5.5, 6.4], while those in the mMITT population were 76.2% (n = 395) and 77.3% (n = 375), respectively (between-group difference −1.1%; 95% CI −7.4, 5.1).[133] A microbiological cure was achieved in 84.3% of doripenem and 84.5% of meropenem recipients in the microbiologically evaluable population.[133] Bacterial eradication rates with doripenem or meropenem were 88% and 84% for E. coli, 78% and 95% for K. pneumoniae, 84% and 79% for B. fragilis, and 85% and 75% for P. aeruginosa.[133]
Two small, noncomparative trials (n = 48 and n = 15) in Japanese patients with IAI (including patients with cholecystitis, cholangitis and hepatic abscesses) found that intravenous doripenem 250 mg two or three times daily, or 500 mg twice daily for 3–14 days achieved clinical response rates (assessed as ‘excellent’ or ‘good’ clinical responses) of 90% and 100%, and bacterial eradication rates of 61% and 55%.[107] The most common pathogens isolated at baseline were P. aeruginosa, K. pneumoniae, E. faecalis, E. coli and B. fragilis.[107]
5.3 Complicated Urinary Tract Infection
The efficacy of intravenous doripenem 250 mg twice daily was compared with that of intravenous meropenem 500 mg twice daily in a Japanese randomized, double-blind trial in adult patients with cUTIs requiring parenteral antibacterial therapy.[135] Intravenous doripenem or meropenem was administered for 5 days in this trial.[135] In another trial (in non-Japanese adult patients with cUTIs requiring parenteral antibacterial therapy), doripenem 500 mg three times daily infused over 30–60 minutes was compared with levofloxacin 250 mg once daily (reported as an abstract and poster).[134] This trial was randomized, double-blind and double-dummy in design, and the planned duration of treatment was 10 days.[134] Patients could be switched to oral levofloxacin tablets (250 mg once daily) after a minimum of 3 days intravenous therapy (nine doses of doripenem). The actual treatment duration was a mean 9.5 days in the doripenem arm and 9.1 days in the levofloxacin arm (intent-to-treat analysis). Patients received a mean 5.4 days of intravenous doripenem or 5.3 days of intravenous levofloxacin before switching to oral levofloxacin (mean of ≈6 days’ treatment).[134] A minority of patients received only intravenous therapy (11% of doripenem and 18% of levofloxacin recipients) for the treatment duration.[134]
Inclusion criteria in the Japanese trial were pyuria of at least five white blood cells per high-power field and bacteriuria of ≥104 CFU/mL, and identifiable underlying urinary tract disease.[135] Most patients were male (72% of the per-protocol population [n = 155]) and ≥75% were aged 60–80 years.[135] At baseline, ≈50% of patients were diagnosed with cystitis[135] and ≈50% were diagnosed with pyelonephritis.[135] Renal function was normal in 83% of patients in the Japanese trial.[135] Patients were included in the trial in non-Japanese patients if a clinical and microbiological diagnosis of cUTI (complicated lower UTI [cLUTI], or complicated or uncomplicated pyelonephritis) was made.[134] Most patients were female (61% of 545 microbiologically evaluable patients) and ≈36% of patients were aged ≥65 years.[134] At baseline, ≈50% of patients were diagnosed with cLUTI[134] and ≈50% were diagnosed with pyelonephritis (severe in 9% of patients).[134] Renal function was normal or mildly impaired (CLCR ≥3.0 L/h) in 87% of patients.[134]
E. coli was the most frequent pathogen isolated at baseline in both studies;[134,135] other organisms isolated included E. faecalis, P. aeruginosa, K. pneumoniae, S. epidermidis, S. marcescens and P. mirabilis.
Both trials were noninferiority studies in which the noninferiority of doripenem to the comparator drug was established if the lower limit of the 95% CI for the difference in the rates of microbiological cure[134] or overall clinical efficacy[135] between the two treatment groups was greater than or equal to −10%. The primary efficacy endpoint in the Japanese trial was overall clinical efficacy (classed as ‘excellent’, ‘moderate’ or ‘poor’, according to criteria proposed by the Japanese UTI Committee in the per-protocol patient population). The per-protocol population was defined as patients who met the protocol-specified disease definition of cUTI, who had an interpretable urine culture at baseline and at the efficacy assessment visit, and who had no entry criteria or protocol violation during treatment that was likely to affect the microbiological outcome. Secondary endpoints included bacteriological response, defined as the percentage of bacterial strains eradicated or presumed eradicated.[135]
The co-primary efficacy endpoints in the other trial were microbiological cure rate (eradication of baseline pathogens [<104 CFU/mL]) at the TOC visit (6–11 days after completing study drug therapy) in the microbiologically evaluable and mMITT populations.[134] The microbiologically evaluable population was defined as patients who met the protocol-specified disease definition of cUTI, who had a study-qualifying pre-treatment urine culture (at least one, but no more than two bacterial uropathogens each at ≥105 CFU/mL) and an interpretable urine culture at the TOC visit, and who had no entry criteria or protocol violation during treatment that was likely to affect the microbiological outcome. The mMITT population included all randomized patients who received at least a partial dose of study therapy and had a study-qualifying pre-treatment urine culture.[134] Secondary endpoints included the microbiological cure rate in microbiologically evaluable patients with E. coli infection at baseline, and microbiological cure rate in the clinically evaluable population (patients who met the criteria for the microbiologically evaluable population but were required to have a clinical outcome assessment, rather than an interpretable urine culture, at the TOC visit).[134]
Doripenem was not inferior to meropenem[135] or levofloxacin[134] in two trials in patients with cUTIs. At the TOC visit, clinical response rates with doripenem or meropenem were ≥88% in the per-protocol population in the Japanese trial[135] (primary endpoint; table XI). The bacteriological response rate with doripenem was noninferior to that with meropenem in the Japanese trial (table XI; between-group difference and 95% confidence intervals not reported), as were clinical efficacy rates judged by the attending urologist (93.4% vs 92.4% [between-group difference and 95% confidence intervals not reported]).[135] Bacterial eradication rates with either agent for Gram-negative or Gram-positive pathogens were >95%.[135]
Efficacy of intravenous doripenem (DOR) in complicated urinary tract infection. Results of two randomized, double-blind, multicentre trials that compared DOR with meropenem (MEM)[135] or levofloxacin (LVX).[134] Study drugs were administered intravenously (IV). One study was available only as an abstract and poster.[134] Response was assessed at the test-of-cure (TOC) visit
In the trial in non-Japanese patients, microbiological cure rates were ≥78% with doripenem or levofloxacin in the microbiologically evaluable and mMITT populations (co-primary endpoint; table XI).[134] Doripenem was also noninferior to levofloxacin in the group of microbiologically evaluable patients with E. coli isolated at baseline in terms of microbiological eradication rates (84.4% vs 87.2% [between-group difference −2.8; 95% CI −10.0, 4.5]). Microbiological eradication rates with doripenem or levofloxacin were 55% and 29% in patients with levofloxacin-resistant (MIC ≥8 mg/L) E. coli, 83% and 63% in K. pneumoniae infection, and 70% and 87% in P. mirabilis infection.[134] Results in the clinically evaluable population at TOC supported the co-primary efficacy outcomes shown in table XI.[134]
A Japanese dose-finding trial of intravenous doripenem in patients with cUTI found that there was no significant difference in efficacy between doripenem 250 or 500 mg administered twice daily.[138] Overall efficacy was seen in 97.4% of patients in the doripenem 250 mg twice daily group and 96.9% of those in the 500 mg twice daily group (primary endpoint). In addition, there were no significant between-group differences for secondary endpoints, including effect on pyuria or bacteriuria and bacteriological response.[138]
Two noncomparative phase II studies demonstrated the efficacy of intravenous doripenem in patients with cUTI.[137,139] In a study evaluating doripenem 125–500 mg twice daily or 500 mg three times daily administered for 3–15 days in patients with respiratory tract infections or cUTIs, overall efficacy was seen in 94% of patients with cUTIs (n = 32), with a bacterial eradication rate of 97% (n = 34).[137] In the other study,[139] which included 6 patients with acute prostatitis, 5 patients with epididymitis and 21 patients with cUTI, doripenem 250 mg two or three times daily or 500 mg twice daily for 3–14 days achieved 84% clinical efficacy judged by attending urologists (27 of 32 patients), and bacterial eradication was 97% (33 of 34 strains isolated at baseline). When assessed by Japanese UTI committee criteria, overall clinical efficacy for cUTIs (n = 16) was 100%. [139]
5.4 Other Infections
Several small, noncomparative studies in Japanese patients have examined the efficacy of intravenous doripenem in a variety of moderate or serious bacterial infections.[108–112,136,140] In these studies, clinical efficacy refers to percentages of patients with ‘excellent’ or ‘good’,[108–111,136] or ‘cured’ or ‘improved’[112,140] clinical responses.
5.4.1 Skin and Skin Structure Infections
Doripenem 250 or 500 mg twice daily for 5–8 days demonstrated good efficacy in the treatment of a range of deep-seated skin infections in a small, noncomparative, Japanese study.[112] A clinical response (cured or improved) was seen at day 7 in all 19 patients (mean age at entry of 50.5 years). The bacteriological response rate was 85% (17 of the 20 pathogens isolated were eradicated).[112] At study entry, ten patients were diagnosed with cellulitis, three with erysipelas, three with lymphangitis, one with lymphadenitis and two with carbuncles. The most common pathogens isolated at baseline were S. aureus, S. pyogenes and K. oxytoca.[112]
5.4.2 Obstetric and Gynaecological Infection
In a small noncomparative study, doripenem showed efficacy in women with moderate or severe gynaecological infection.[108] Intravenous doripenem 250 mg administered two or three times daily, or 500 mg administered twice daily for 3–14 days (mean of 7.2 days) achieved clinical efficacy (excellent or good response) in 89% of women (n = 54; mean age of 41 years) enrolled in this study. Bacteriological eradication was evident in 80% of microbiologically evaluable patients (24 of 30 patients).[108] Gynaecological infections included in this study were parametritis (18 patients), pelvic peritonitis (14 patients), uterine adnexitis (10 patients), intrauterine infection (9 patients) and pouch of Douglas abscess (3 patients). Infections were severe in 41 patients and moderate in the remaining 13 patients.[108] Pathogens were isolated at baseline in 33 patients, and these included S. aureus, Group B Streptococcus, E. faecium, E. coli, P. aeruginosa and various anaerobes. Two-thirds of patients who were microbiologically evaluable (21 of 30 patients) had polymicrobial infection.[108]
5.4.3 Sepsis and Endocarditis
Intravenous doripenem 500 mg two or three times daily administered for 3–14 days in patients with sepsis (n = 9) and for 28 days in patients with infective endocarditis (n = 2) was associated with clinical cure and eradication of bacterial pathogens in all patients in a small study.[140] Patients were aged 59–79 years and had APACHE II scores ranging from 6 to 16.[140] Sepsis was secondary to urological infection in seven patients and surgery in two patients and, where identified, causative pathogens were E. coli and K. pneumoniae.[140] The causative organisms in patients with infective endocarditis were Streptococcus spp. (S. sanguis and S. vestibularis [MICs not reported]).[140]
5.4.4 Ear, Nose and Throat Infections
In 12 adult patients with moderate to severe otitis media or tonsillitis (including peritonsillar abscess), intravenous doripenem achieved clinical efficacy in 92% of patients (11 of 12 patients) and a bacteriological eradication rate of 92% (11 of 12 organisms).[109] Patients were aged 23–70 years and 8 of 12 patients were described as having severe, rather than moderate, infection. Doripenem 250 mg two or three times daily, or 500 mg twice daily was administered for 6–7 days. The most common pathogens isolated at baseline were S. aureus, S. pyogenes and P. aeruginosa.[109]
In a study of doripenem 250 mg two or three times daily, or 500 mg twice daily for 3–14 days in patients with a range of serious infections (n = 105), clinical efficacy was achieved at treatment end in both of the patients who had peritonsillar abscess.[136]
5.4.5 Dental and Oral Surgical Infection
Intravenous doripenem showed efficacy in a small study in 24 patients with osteitis or cellulitis associated with dental or oral infection.[110] Clinical efficacy at end of treatment was achieved in 100% of patients and bacteriological eradication was evident in 96% of patients (23 of 24 patients).[110] Doripenem 250 mg two or three times daily, or 500 mg twice daily was administered for 3–7 days. Most infections were polymicrobial, and included a mixture of aerobic Gram-positive or Gram-negative bacteria and anaerobes. The most common pathogens isolated at baseline were Streptococcus spp. (S. sanguis, S. mitis, S. oralis and S. constellatus).[110]
5.4.6 Ophthalmic Infection
Doripenem penetrates aqueous humor, evidenced by concentrations of the drug of 0.16–0.87 mg/L within 1–2 hours of starting an infusion of a 250 mg intravenous dose.[111] Consequently, doripenem has been studied in patients with ophthalmic infection. In 15 patients with ophthalmic infection (eight patients had pathogens isolated at baseline), intravenous doripenem achieved a clinical response in 100% of patients and bacteriological eradication of all causative pathogens.[111] Doripenem 250 mg two or three times daily, or 500 mg twice daily was administered for 5–12 days (no local treatment was administered). Ten patients were diagnosed with corneal ulcer; four patients had an orbital infection and one patient had endophthalmitis.[111] The most common organisms isolated at baseline were Corynebacterium spp. (two corneal ulcer and one endophthalmitis patients) and P. aeruginosa (two corneal ulcer patients).[111]
6. Tolerability
Discussion in this section is based on data from the seven randomized, multicentre, comparative clinical studies[126,127,129,131–135] reviewed in section 5, supplemented by data from the Japanese[12] and the US[13] prescribing information.
Doripenem was generally well tolerated in both Japanese and non-Japanese patients in clinical trials,[12,13] and adverse events were mild or moderate in severity.[126,127,129,131,132,134,135] In individual trials, treatment-emergent adverse events (regardless of causality) were reported in 35–83% of doripenem recipients,[127,131,132,134] 27–78%[127,131,132] of meropenem recipients and 60% of levofloxacin recipients.[134] In two trials in patients with cIAIs[131,132] and a trial in patients with cUTIs including pyelonephritis,[134] the most common adverse events occurring in ≥1% of patients who received doripenem, meropenem or levofloxacin, regardless of causality, were headache, nausea, diarrhoea, anaemia, phlebitis and rash (figure 3).[13]
Tolerability of intravenous doripenem (DOR) in adult patients (pts) with complicated urinary tract infections (cUTIs) or complicated intra-abdominal infections (cIAIs). (a) Combined results[13] of two trials[131,132] in which patients with cIAIs received 5–14 days of intravenous DOR 500 mg every 8 hours infused over 60 min (n = 477) or meropenem (MEM) 1 g every 8 hours administered as a bolus over 3–5 min (n = 469) and (b) results[13] of a trial[134] in which patients with cUTIs were treated for a total of 10 days with intravenous DOR 500 mg every 8 hours (n = 376) or levofloxacin (LVX) 250 mg once daily (n = 372), each administered as a 60-min infusion, with the option after 3 days (after at least nine doses of DOR or at least three doses of LVX) of switching to oral LVX 250 mg once daily for the remainder of the treatment period. Adverse events occurring in ≥1% of patients in any treatment arm, regardless of cause.
Treatment-related adverse events were uncommon (incidence of 4.4%; 37 of 835 patients) in Japanese doripenem recipients (835 individuals evaluable at the time of approval in Japan),[12] and diarrhoea (0.7%) and rash (0.6%) were the most frequent adverse events reported. The incidence of treatment-related adverse events with doripenem or comparator drugs did not differ significantly in the individual randomized, comparative trials,[126,127,129,133–135] and the most frequent treatment-related adverse events in any treatment arm were gastrointestinal (mainly nausea or diarrhoea), headache and phlebitis.
Severe treatment-related adverse events, such as hypersensitivity reactions or Clostridium difficile colitis, were rare (both at frequency of <1%) in these three trials and few patients discontinued doripenem therapy because of an adverse event (0.2% due to nausea, 0.1% due to vulvomycotic infection and 0.1% due to rash).[13] No treatment-related serious adverse events were reported in the individual randomized trials.[127,131,132,134,135]
Laboratory abnormalities were generally infrequent in clinical trials in non-Japanese patients with cIAI, cUTI or nosocomial pneumonia.[129,131,132,134] For example, in the cIAI and cUTI trials, elevated liver enzymes occurred in 1–2% of doripenem recipients, 3% of ciprofloxacin recipients and 3% of meropenem recipients.[13] Abnormal laboratory results were reported in 23.8% of Japanese individuals (818 evaluable) who had received doripenem up to the time of approval of the drug in Japan.[12] The most frequent abnormalities were increased ALT (12.7%) or AST (9.7%) levels.[12] In the randomized comparisons between doripenem and meropenem in Japanese patients, the incidence and type of treatment-related abnormal laboratory results did not differ between the doripenem and meropenem groups.[127,135]
Seizures were reported in 0–1.3% of doripenem, 0% of meropenem, 0.3% of levofloxacin, 2.7% of piperacillin/tazobactam and 3.8% of imipenem/cilastin recipients in the randomized, comparative trials;[126,127,129,131,132,134,135] however, apart from one incident in an imipenem/cilastin recipient,[129] all were thought to be unrelated to the study medication.[126,129,134] Few patients died in any of these clinical trials, and none of the deaths reported were deemed related to the study medication.[126,127,129,131,132,134,135]
Treatment-emergent anaphylaxis, interstitial pneumonia, Stevens Johnson syndrome, seizure and toxic epidermal necrolysis have been observed with doripenem therapy during postmarketing surveillance, although frequency and causality have not been established.[13] Doripenem is currently contraindicated in patients who have demonstrated anaphylactic reactions to β-lactam agents or who have a known sensitivity to carbapenems.[12,13]
7. Dosage and Administration
Doripenem is indicated in Japan for use as a single agent in the treatment of adult patients with a wide range of infections (see table XII) caused by susceptible strains of Staphylococcus spp., Streptococcus spp., Enterococcus spp. (except E. faecium), M. catarrhalis, E. coli, Citrobacter spp., Klebsiella spp., Enterobacter spp., Serratia spp., Proteus spp., M. morganii, Providencia spp., H. influenzae, P. aeruginosa, Acinetobacter spp., Peptostreptococcus spp., Bacteroides spp. and Prevotella spp.[12]
Approved indications for intravenous doripenem in Japan[12]
The recommended dosage regimen in Japan is 250 mg two or three times daily (infused over 30–60 minutes), although the dosage can be increased, depending on patient age and symptoms.[12] The maximum individual dose is 500 mg, and the maximum daily dosage is 1.5 g/day.[12]
In the US, doripenem is indicated as a single agent for the treatment of adult patients (aged ≥18 years) with cIAIs or cUTIs, including pyelonephritis, caused by susceptible strains of designated pathogens.[13] For cIAIs, these pathogens are B. caccae, B. fragilis, B. thetaiotaomicron, B. uniformis, B. vulgatus, E. coli, K. pneumoniae, P. micros, P. aeruginosa, S. constellatus and S. intermedius; for cUTIs, including pyelonephritis, they are A. baumannii, E. coli (including patients with concurrent bacteraemia), K. pneumoniae, P. mirabilis and P. aeruginosa.[13]
The recommended dosage regimen for doripenem in the US is 500 mg every 8 hours (infused over 60 minutes) for 5–14 days (patients with cIAIs) or 10 days (patients with cUTIs, including pyelonephritis).[13] In patients with cUTIs, including pyelonephritis, and concurrent bacteraemia, doripenem can be administered for up to 14 days.[13] Patients may be switched to an orally administered antibacterial agent after 3 days of doripenem therapy upon the demonstration of clinical improvement.[13]
Doripenem is approved in the EU for use in adult patients with nosocomial pneumonia (including VAP), cIAIs and cUTIs.[14] The recommended dosage in the EU is 500 mg every 8 hours, for 5–14 days, infused over 1 hour in patients with cIAIs or cUTIs (including pyelonephritis) and over 1 or 4 hours in patients with nosocomial pneumonia (including VAP).[14]
Doripenem is formulated as a powder for reconstitution with 100 mL of physiological saline (sodium chloride 0.9%) in Japan,[12] or with sterile water or sodium chloride 0.9% to produce a suspension containing 500 mg in 10 mL then diluted with 100 mL of sodium chloride 0.9% or dextrose 5% in the US and EU.[13,14] At a controlled room temperature, doripenem solution is stable for up to 4 hours when reconstituted with dextrose and up to 12 hours when reconstituted with physiological saline. At a refrigerated temperature of 2–8°C protected from light, the drug is stable for up to 24 hours in a dextrose solution and up to 72 hours in physiological saline.[14]
Dosage adjustment is recommended for patients with renal impairment.[12–14] Doripenem is not recommended in patients aged <18 years because of a lack of efficacy and tolerability data.[12–14]
The local manufacturer’s prescribing information should be consulted for other contraindications, drug interactions, monitoring requirements, precautions, details of dosage adjustments in renal impairment and use in other special patient populations.
8. Place of Doripenem in the Management of Bacterial Infections
Prompt and appropriate use of antimicrobial therapy, including using local microbiological data in selecting appropriate treatment,[143–145] is important in reducing morbidity and mortality associated with serious bacterial infections.[2,144,146]
Among the antibacterial agents commonly used in this setting are the carbapenems, a class of broad-spectrum β-lactam antibacterials identified in the 1970s.[1] In addition, panipenem/betamipron[11] (approved in Japan, China and Korea) and biapenem[10] (approved in Japan) have been available for several years.[1] Doripenem was approved in Japan in 2005 for use in a wide range of serious infections due to Gram-negative, Gram-positive or anaerobic pathogens,[12] in the US in late 2007 for use in adult patients with cIAIs or cUTIs,[13] and in the EU in 2008 in adult patients with nosocomial pneumonia (including VAP), cIAIs and cUTIs.[14] Doripenem is currently undergoing pre-approval review for use in nosocomial pneumonia, including VAP, in the US[147] and for use in adult patients with cIAIs, cUTIs or nosocomial pneumonia, including VAP, in other countries worldwide.[147]
Imipenem/cilastin or meropenem are well established, appropriate choices for the empirical treatment of serious bacterial infections in a hospital setting, including nosocomial infections, because of their broad spectrum of antibacterial activity and good efficacy.[1,144] Meropenem is administered every 8 hours as a 500 mg dose infused over 15–30 minutes (skin and skin structure infections), or a 1000 mg dose administered as a 15- to 30-minute infusion or as an intravenous bolus injection (in cIAIs),[148] while imipenem/cilastin is usually administered every 6 or 8 hours as a 500 mg dose infused over 40–60 minutes (in moderate or severe infections with susceptible organisms).[149] In general, imipenem has slightly greater activity against Gram-positive organisms than meropenem, while meropenem is slightly more active than imipenem against Gram-negative organisms.[1] Nevertheless, both have broad-spectrum activity, including against non-fermenting Gram-negative bacteria. Doripenem, although more recently approved, is considered a viable alternative to these agents because of its similar spectrum of activity and clinical efficacy to imipenem against Gram-positive organisms and to meropenem against Gram-negative organisms,[144] and good activity against P. aeruginosa and other non-fermentative Gram-negative bacilli.[1,68] Importantly, doripenem activity against Gram-negative pathogens is generally 2- to 4-fold greater than that of imipenem (section 2.2.1).[144] Doripenem is usually administered every 8 hours as a 250 mg (in Japan only) or 500 mg dose infused over 30–60 minutes,[12,13] although more aggressive administration scheduled may be required in critically ill patients with altered pharmacokinetics (e.g. with increased Vd).[144] In contrast, ertapenem is not suited for empirical use in nosocomial infections because of an absence of activity against non-fermentative Gram-negative pathogens.[8] Doripenem (like meropenem,[5–7,150] ertapenem[8,9] or biapenem,[10] but unlike imipenem[4] or panipenem[11]) is stable to the human renal enzyme DHP-1 (section 2.1) and does not need to be coadministered with a DHP-1 inhibitor to achieve clinical efficacy (e.g. cilastin plus imipenem[4]) or to reduce nephrotoxic potential (e.g. betamipron plus panipenem[11]). Doripenem is also resistant to inactivation by most β-lactamases, including ESBLs (section 2).
Like meropenem[150] or imipenem,[4] doripenem has a short t½β (section 3); consequently, doripenem requires administration two or three times daily, usually as a 30- to 60-minute infusion (section 7). Unlike the other available carbapenems,[144] doripenem is stable at room temperature for up to 12 hours when reconstituted with normal saline (section 7), which means that it may have potential for prolonged infusion regimens (e.g. the 4-hour infusion used in a clinical trial[129] in VAP [section 5.1.1]), thus achieving the T > MICfree targets required in more difficult-to-treat nosocomial infections.[143]
Plasma concentrations of doripenem are estimated to achieve an optimal bactericidal pharmacodynamic target attainment against most pathogens associated with serious bacterial infections, including nosocomial pneumonia, cUTI or cIAI (section 4). The drug has shown good penetration into a wide range of tissues, including lung, intra-abdominal, and head and neck tissues, and peritoneal fluid, and demonstrates low serum protein binding (section 3).
Doripenem demonstrated in vitro activity against a wide range of Gram-negative, Gram-positive and anaerobic organisms associated with serious or nosocomially acquired infections (section 2.2), and has rapid, time-dependent bactericidal activity against clinically important Gram-negative and Gram-positive pathogens (including P. aeruginosa, E. coli and S. aureus) and various anaerobes. In addition, doripenem has an in vitro PAE of ≈2 hours against P. aeruginosa and S. aureus (section 2.2.5). This broad spectrum of activity means that doripenem is suitable for use as an empirical treatment for serious bacterial infections, such as serious lower respiratory infections (including nosocomial pneumonia, VAP or severe CAP), cIAI, cUTIs, gynaecological and obstetric infections and sepsis, especially as these conditions are often polymicrobial, including mixed aerobic/anaerobic, infections. Like meropenem[150] or biapenem,[10] doripenem had a low propensity for inducing seizures in animal studies (section 2.5) and was not associated with seizures in clinical trials (section 6).[44] However, further investigation in a clinical setting is required to confirm these results.
Numerous randomized, phase III clinical trials have shown doripenem to be an effective and well tolerated treatment in a wide range of serious bacterial infections (section 5). Doripenem was as effective as comparator antibacterials, including meropenem in complicated lower respiratory infections, cIAIs and cUTIs, imipenem/cilastin or piperacillin/tazobactam in nosocomial pneumonia (including VAP) and levofloxacin in cUTIs (section 5.1, section 5.2 and section 5.3). Doripenem also demonstrated efficacy in a range of other infections, including cSSSI, obstetric or gynaecological infections, sepsis and endocarditis, severe ENT, dental or oral surgical infections and severe ophthalmic infections (section 5.4).
Pharmacoeconomic analyses of doripenem, including analyses based on efficacy and hospital resource utilization data discussed in section 5, would be useful in determining the cost effectiveness of the drug, relative to other carbapenems or conventional combination antibacterial treatments in patients with serious bacterial infections.
International treatment guidelines[151–157] support the use of carbapenems, such as imipenem/cilastin or meropenem, as an option in the initial treatment of nosocomial pneumonia and/or severe CAP (in combination with ciprofloxacin where there is a risk of P. aeruginosa),[153–155] cUTIs,[156] incisional surgical site infection after intestinal or genital tract surgery,[151] and severe IAIs,[152] and list carbapenems in a range of first-line regimens in the treatment of polymicrobial necrotizing infections of the skin, fascia and muscle, or as intravenous options in infections after a human or an animal bite.[151] These guidelines were published prior to the approval of doripenem outside of Japan, and thus do not include doripenem among the carbapenems recommended. Japanese guidelines[158,159] support the use of doripenem, meropenem or imipenem/cilastin as a second-line option in the treatment of moderate or severe cholangitis[158] and use of carbapenems in severe pneumococcal pneumonia requiring inpatient treatment or as empirical therapy in combination with new quinolones, tetracyclines or macrolides in patients with severe CAP who require admission to the intensive care unit.[159]
Increased and/or inappropriate use of broad-spectrum antibacterial agents, such as the third-generation cephalosporins, in serious bacterial infections has resulted in a global increase in resistant bacterial strains, including ESBL- and AmpC-producing Enterobacteriaceae, and multidrug-resistant P. aeruginosa.[2,146,160] Because of their broad spectrum of activity, carbapenems are considered first-line options in the empirical treatment of serious infections that may be associated with ESBL-producing Enterobacteriaceae or P. aeruginosa.[160] Importantly, doripenem retains activity against ESBL- and AmpC-producing Enterobacteriaceae, with little or no increase in MIC90 values when compared with wild-type isolates, has no inoculum effect (section 2.2 and section 2.3) and a low propensity to select for resistance (section 2.3).
Antimicrobial resistance to the non-fermentative Gram-negative pathogens Acinetobacter spp. and P. aeruginosa is increasing worldwide, and multidrug-resistant isolates of P. aeruginosa and A. baumanii are often associated with nosocomial infection.[161–164] Doripenem showed in vitro activity against ceftazidime- or imipenem-susceptible, or non-multidrug-resistant P. aeruginosa isolates in all geographic regions, evidenced by MIC90 values of 0.2–12.5 mg/L and, in some studies, susceptibility rates of 100% (section 2.2). Nevertheless, like other carbapenems, doripenem activity against resistant P. aeruginosa isolates was more limited, with susceptibility rates of ≤40% and MIC90 values of 8–64 mg/L (section 2.2). Doripenem activity against A. baumanii (including ceftazidime intermediate/resistant isolates) was limited in Europe and the Americas, with a susceptibility rate of 76–78% and MIC90 values of 1–32 mg/L; however, lower MIC90 values for Acinetobacter spp. were reported in Japan (0.78–3.13 mg/L) [section 2.2]. As a consequence of its broad spectrum of in vitro activity against Gram-negative bacteria, including those pathogens resistant to other antibacterials, doripenem has potential as a new option in the empirical treatment of patients with severe nosocomial infections, including in settings where there are increased rates of multidrug-resistant Gram-negative bacteria.[144,165]
Of interest, penicillin- or macrolide-resistant S. pneumoniae isolates are more common in Asia (including Japan) than in Western countries. [166] In Japan, >50% of macrolide-resistant isolates are erm B-positive, which is usually associated with higher MICs than mef A-positive isolates.[159] Doripenem showed good in vitro activity against S. pneumoniae, including ceftriaxone-resistant, penicillin-intermediate or -resistant or multidrug-resistant isolates (sections 2.2 and 2.3).
Methicillin or oxacillin-resistant S. aureus, E. faecium and S. maltophilia (section 2.2) are inherently resistant to doripenem and other currently available carbapenems.[1] Although the prevalence of acquired metallo-β-lactamases and carbapenemases is increasing (section 2.3),[78,88] carbapenem resistance remains uncommon and Enterobacteriaceae resistance to carbapenems is rare (section 2.3).[47,160] However, it is important that doripenem (and other carbapenems) are used appropriately as empirical treatment to limit the likelihood of increasing future resistance.[2] Ideally, doripenem should only be used in a hospital setting in severe infections for the shortest possible duration.
As mentioned in section 4, the pharmacodynamic/pharmacokinetic parameter that best correlates with carbapenem bactericidal activity (and therefore efficacy) is T > MICfree, and a T > MICfree of 30–40% is adequate for carbapenem efficacy. Of interest, in animal model of infections, increasing the doripenem dose administration frequency or lengthening the infusion duration was associated with a prolonged T > MICfree and enhanced bactericidal activity, compared with increasing the dose per administration (section 4). This is particularly important when considering pathogens with increased MICs, including P. aeruginosa. Unlike meropenem[148] or imipenem[149] (which maintain potency for only 2[148] or 4[149] hours at room temperature once reconstituted), doripenem solution is stable for up to 12 hours at a controlled room temperature when reconstituted with physiological saline (section 7). Consequently, doripenem is suitable for prolonged infusion regimens, such as the 4-hour infusion used in a trial in patients with VAP[129] (section 5.1.1).
In conclusion, doripenem has a broad spectrum of in vitro activity against Gram-positive and Gram-negative pathogens, including ESBL- and AmpC-producing Enterobacteriaceae, and anaerobic bacteria. The drug also has a low propensity to select for resistance and is suitable for the prolonged infusions that may be required to achieve pharmacodynamic/pharmacokinetic targets for bactericidal activity (and therefore efficacy) against pathogens with increased MICs. Doripenem is at least as effective as other antibacterial agents, including meropenem, imipenem/cilastin, piperacillin/tazobactam or levofloxacin, in the treatment of a variety of serious bacterial infections (e.g. complicated lower respiratory infections, nosocomial pneumonia [including VAP], cIAIs and cUTIs), and is well tolerated. Thus, doripenem is a valuable addition to the options available for the empirical treatment of hospitalized patients with serious bacterial infections.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Zhanel GG, Wiebe R, Dilay L, et al. Comparative review of the carbapenems. Drugs 2007; 67(7): 1027–52
Kollef M. Appropriate empirical antibacterial therapy for nosocomial infections: getting it right the first time. Drugs 2003; 63(20): 2157–68
Shimada J, Yamaguchi K, Shiba T, et al. A new carbapenem antibiotic for injection: characteristics of doripenem [in Japanese]. Jpn J Antibiot 2005 Dec; 58(6): 489–506
Balfour JA, Bryson HM, Brogden RN. Imipenem/cilastin: an update of its antibacterial activity, pharmacokinetics and therapeutic efficacy in the treatment of serious infections. Drugs 1996 Jan; 51(1): 99–136
Lowe MN, Lamb HM. Meropenem: an updated review of its use in the management of intra-abdominal infections. Drugs 2000 Sep; 60(3): 619–46
Hurst M, Lamb HM. Meropenem: a review of its use in patients in intensive care. Drugs 2000 Mar; 59(3): 653–80
Wiseman LR, Wagstaff AJ, Brogden RN, et al. Meropenem: a review of its antibacterial activity, pharmacokinetic properties and clinical efficacy. Drugs 1995 Jul; 50(1): 73–101
Keating GM, Perry CM. Ertapenem: a review of its use in the treatment of bacterial infections. Drugs 2005; 65(15): 2151–78
Curran M, Simpson D, Perry C. Ertapenem: a review of its use in the management of bacterial infections. Drugs 2003; 63(17): 1855–78
Perry CM, Ibbotson T. Biapenem. Drugs 2002; 62(15): 2221–34; discussion 2235
Goa KL, Noble S. Panipenem/betamipron. Drugs 2003; 63(9): 913–25; discussion 926
Shionogi and Co Ltd, Osaka. Prescribing information. Doripenem hydrate (Finibax®) 0.25g IV solution [in Japanese]. 2005 Jul
Johnson & Johnson Pharmaceutical Research Development LLC, Raritan (NJ). Doribax™ [doripenem] prescribing information 2007 Oct [online]. Available from URL: http://www.doribax.com/doribax/shared/pi/doribax.pdf#zoom=100 [Accessed 2008 Apr 14]
European Medicines Authority. Summary of product characteristics: Doribax [online]. Available from URL: http://www.emea.europa.eu/humandocs/Humans/EPAR/doribax/doribax.htm [Accessed 2008 Aug 5]
Davies TA, Shang W, Bush K, et al. Affinity of doripenem and comparators to penicillin-binding proteins in Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2008 Apr; 52(4): 1510–2
Fujimura T, Kimura Y, Yoshida I, et al. In vitro antibacterial activity of doripenem, a novel parenteral carbapenem [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 57–70
Davies TA, Bush K, Flamm RK. Differences in Escherichia coli and Pseudomonas aeruginosa cell morphology after exposure to doripenem, imipenem, and meropenem [abstract no. A-025]. 107th General Meeting of the American Society for Microbiology; 2007 May 21–25; Toronto (ON)
Mori M, Hikida M, Nishihara T, et al. Comparative stability of carbapenem and penem antibiotics to human recombinant dehydropeptidase-I [letter]. J Antimicrob Chemother 1996 May; 37(5): 1034–6
Yamano Y, Kawai Y, Yutsudo T. Stability of doripenem against human renal dehydropeptidase-I [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 92–5
Turnidge JD, Bell JM, Fritsche TR, et al. Doripenem activity tested against Gram-negative pathogens in the Asia-Pacific (APAC) region: report from the SENTRY Antimicrobial Surveillance Program, 2006 [abstract no. E260 plus poster]. 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Evangelista AT, Yee YC, Pillar CM, et al. Surveillance profiling of doripenem (DOR) activity against Pseudomonas aeruginosa (PA) isolated from inpatients (IP) and ICU patients (ICU); results of the TRUST surveillance initiative [abstract no. 523]. 45th Annual Infectious Diseases Society of America; 2007 Oct 4–7; San Diego (CA), 159
Fritsche TR, Sader HS, Strabala P, et al. Antimicrobial activities of doripenem and other carbapenems tested against Pseudomonas aeruginosa, other non-fermentative bacilli and Aeromonas spp [abstract no. E-0222 plus poster]. 46th Inter-science Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA), 177
Fritsche TR, Sader HS, Strabala P, et al. Activity of doripenem tested against an international collection of ESBL- and AmpC-producing Enterobacteriaceae [abstract no. E-0219 plus poster]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA), 176
Fritsche T, Sader HS, Strabala P, et al. Spectrum and activity of doripenem, an investigational carbapenem, tested against bacterial pathogens recovered from patients hospitalized with pneumonia (Europe 2004–2006) [abstract no. 2087 plus poster]. 17th European Congress of Clinical Microbiology and Infectious Diseases; 2007 Mar 31–Apr 3; Munich
Fritsche TR, Sader HS, Jones RN. Geographic variations in the activity of doripenem and other broad-spectrum agents: results from an international surveillance program (2003-2005) [abstract no. C2-1836 plus poster]. 46th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA)
Fritsche TR, Jones RN. Antimicrobial activity of broad-spectrum β-lactams, including doripenem, tested against bloodstream infection isolates: a global surveillance report (2003-2005) [abstract no. 200]. 44th Annual Infectious Diseases Society of America; 2006 Oct 12–15; Toronto (ON), 81
Fritsche T, Sader HS, Strabala P, et al. Variations and trends in the activity of doripenem and other broad-spectrum agents against leading bacterial pathogens: results from a European surveillance program (2003–2006) [abstract no. P 2088 plus poster]. 17th European Congress of Clinical Microbiology and Infectious Diseases; 2007 Mar 31–Apr 3; Munich
Aranza M, Pillar C, Draghi D, et al. Baseline surveillance profile of doripenem against key Gram-negative pathogens encountered in Europe [abstract no. 1664 plus poster]. 17th European Congress of Clinical Microbiology and Infectious Diseases; 2007 Mar 31–Apr 3; Munich
Brown NP, Jones ME, Draghi DC, et al. Baseline surveillance profile of doripenem against key Gram-negative pathogens encountered in the United States [abstract no. E221 plus poster]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA), 177
Brown NP, Jones ME, Draghi DC, et al. Profile of doripenem activity against Gram-positive pathogens: results of a 2005–2006 surveillance initiative [abstract no. E220 plus poster]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA), 176–7
Pillar CM, Aranza MK, Shah D, et al. Analysis of doripenem activity, relative to other carbapenems, against target Gram-negative pathogens isolated from specific infection sites [abstract no. E-262 plus poster]. 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL), 185
Fritsche TR, Stilwell MG, Jones RN. Antimicrobial activity of doripenem (S-4661): a global surveillance report (2003). Clin Microbiol Infect 2005 Dec; 11(12): 974–84
Kaniga K, Prokimer P, Llorens L, et al. Prevalence of extendedspectrum beta-lactamase (ESBL) and fluoroquinolone-resistant isolates from phase 3 trials of nosocomial pneumonia and their susceptibility to doripenem (DOR) [abstract no. E265]. 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Queenan AM, Shang W, Amsler K, et al. Characterization of ESBLs from Enterobacteriaceae collected during doripenem clinical trials [abstract no. C2-1519]. 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Kaniga K, Umeh O, Llorens L, et al. Activity of doripenem against isolates from phase 3 trials of complicated intra-abdominal infections [abstract no. E-264 plus poster]. 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Kaniga K, Redman R, Llorens L, et al. Prevalence of extendedspectrum beta-lactamase producers (ESBLs) and fluoroquinolone-resistant isolates from phase 3 trials of complicated urinary tract infections (UTIs) including pyelonephritis [abstract no. E-925 plus poster]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Goldstein EJ, Citron DM, Merriam CV, et al. In vitro activities of doripenem and six comparator drugs against 423 aerobic and anaerobic bacterial isolates from infected diabetic foot wounds. Antimicrob Agents Chemother 2008 Feb; 52(2): 761–6
Aranza MK, Pillar CM, Shah D, et al. Stratified analysis of doripenem activity against Enterobacteriaceae and Pseudomonas aeruginosa according to patient location: inpatients and ICU patients [abstract no. E-263 plus poster]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Brown SD, Traczewski MM. Comparative in vitro antimicrobial activity of a new carbapenem, doripenem: tentative disc diffusion criteria and quality control. J Antimicrob Chemother 2005 Jun; 55(6): 944–9
Fritsche TR, Johnson D, Jones RN. Antimicrobial activity of doripenem against multi-drug resistant Pseudomonas aeruginosa and Streptococcus pneumoniae [abstract no. E2014]. 44th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2004 Oct 30–Nov 2; Washington DC, 175
Ge Y, Wilder MA, Sahm DF, et al. In vitro antimicrobial activity of doripenem, a new carbapenem. Antimicrob Agents Chemother 2004 Apr; 48(4): 1384–96
Hecht DW, Galang MA, Sambol SP, et al. In vitro activities of 15 antimicrobial agents against 110 toxigenic Clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob Agents Chemother 2007 Aug; 51(8): 2716–9
Credito KL, Ednie LM, Appelbaum PC. Comparative antianaerobic activities of doripenem determined by MIC and time-kill analysis. Antimicrob Agents Chemother 2008 Jan; 52(1): 365–73
Jones RN, Huynh HK, Biedenbach DJ. Activities of doripenem (S-4661) against drug-resistant clinical pathogens. Antimicrob Agents Chemother 2004 Aug; 48(8): 3136–40
Jones RN, Huynh HK, Biedenbach DJ, et al. Doripenem (S-4661), a novel carbapenem: comparative activity against contemporary pathogens including bactericidal action and preliminary in vitro methods evaluations. J Antimicrob Chemother 2004 Jul; 54(1): 144–54
Mushtaq S, Ge Y, Livermore DM. Doripenem versus Pseudomonas aeruginosa in vitro: activity against characterized isolates, mutants, and transconjugants and resistance selection potential. Antimicrob Agents Chemother 2004 Aug; 48(8): 3086–92
Mushtaq S, Ge Y, Livermore DM. Comparative activities of doripenem versus isolates, mutants, and transconjugants of Enterobacteriaceae and Acinetobacter spp. with characterized β-lactamases. Antimicrob Agents Chemother 2004 Apr; 48(4): 1313–9
Traczewski MM, Brown SD. In vitro activity of doripenem against Pseudomonas aeruginosa and Burkholderia cepacia isolates from both cystic fibrosis and non-cystic fibrosis patients. Antimicrob Agents Chemother 2006 Feb; 50(2): 819–21
Wexler HM, Engel AE, Glass D, et al. In vitro activities of doripenem and comparator agents against 364 anaerobic clinical isolates. Antimicrob Agents Chemother 2005 Oct; 49(10): 4413–7
Davies TA, Shang W, Bush K, et al. Activity of doripenem and comparator β-lactams against US clinical isolates of Streptococcus pneumoniae with defined mutations in the penicillin-binding domains of pbp 1a,pbp2b and pbp2x [letter]. J Antimicrob Chemother 2008 Mar; 61(3): 751–3
Jones RN, Sader HS, Fritsche TR. Comparative activity of doripenem and three other carbapenems tested against Gramnegative bacilli with various β-lactamase resistance mechanisms. Diagn Microbiol Infect Dis 2005 May; 52(1): 71–4
Yamano Y, Nishikawa T, Fujimura T, et al. Occurrence of PER-1 producing clinical isolates of Pseudomonas aeruginosa in Japan and their susceptibility to doripenem. J Antibiot (Tokyo) 2006 Dec; 59(12): 791–6
Nomura S, Nagayama A. In vitro antibacterial activity of S-4661, a new parenteral carbapenem, against urological pathogens isolated from patients with complicated urinary tract infections. J Chemother 2002 Apr; 14(2): 155–60
Mikamo H, Izumi K, Hua YX, et al. In vitro and in vivo antibacterial activities of a new injectable carbapenem, S-4661, against gynaecological pathogens. J Antimicrob Chemother 2000 Sep; 46(3): 471–4
Watanabe A, Takahashi H, Kikuchi T, et al. Comparative in vitro activity of S-4661, a new parenteral carbapenem, and other antimicrobial agents against respiratory pathogens. Chemotherapy 2000 May 30; 46(3): 184–7
Shimauchi C, Kaneko K, Sato Y, et al. Comparative basic study of carbapenems antibiotics against Pseudomonas aeruginosa [in Japanese]. Jpn J Chemother 2005; 53(12): 732–40
Kuwahara-Arai K, Hiramatsu K. In vitro activity of doripenem against Gram-positive and Gram-negative bacteria isolates [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 17–23
Miwa H, Kimura Y, Jinushi Y, et al. Antipseudomonal activity of doripenem, a novel parenteral carbapenem antibiotic [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 80–91
Okamoto R, Nakano R, Sato Y, et al. Antibacterial activity of doripenem against β-lactamase-producing strains [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 47–51
Tanaka K, Watanabe K. In vitro activity of doripenem, a lβmethylcarbapenem, against anaerobic bacteria [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 24–31
Yoshida I, Sugimori G, Higashiyama I, et al. Surveillance of susceptibility of clinical isolates of various bacterial species to antibacterial agents: antimicrobial activity against Gram-negative bacteria isolated in 2000 [in Japanese]. Jpn J Chemother 2003; 51(4): 209–32
Masuda N, Sakagawa E, Ohya S, et al. Hypersusceptibility of the Pseudomonas aeruginosa nfxB mutant to β-lactams due to reduced expression of the AmpC β-lactamase. Antimicrob Agents Chemother 2001 Apr; 45(4): 1284–6
Tsuji M, Furuya N, Matsumoto T, et al. In vitro and in vivo antibacterial activity of DRPM, a new carbapenem [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 1–16
Mikamo H, Tamaya T. Antimicrobial susceptibility and pharmacokinetics of doripenem in the field of obstetrics and gynecology [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 286–92
Sugihara K, Tateda K, Shibuya R, et al. In vitro activity of CS-023 (RO4908463) against recent clinical isolates of Gram-positive and -negative pathogens in Japan [abstract no. E271]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Kobayashi Y, Sumitani Y, Sugita K, et al. Antimicrobial activity of meropenem against main bacterial species isolated from patient blood in 2006 [in Japanese]. Jpn J Antibiot 2007 Dec; 60(6): 378–86
Chen Y, Garber E, Zhao Q, et al. In vitro activity of doripenem (S-4661) against multidrug-resistant Gram-negative bacilli isolated from patients with cystic fibrosis. Antimicrob Agents Chemother 2005 Jun; 49(6): 2510–1
Jones RN, Sader HS, Fritsche TR, et al. Selection of a surrogate β-lactam testing agent for initial susceptibility testing of doripenem, a new carbapenem. Diagn Microbiol Infect Dis 2007 Dec; 59(4): 467–72
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; eighteenth informational supplement. M100-S18 Vol.28 No 1. Wayne (PA): Clinical and Laboratory Standards Institute, 2008 Jan
Nishino T, Otsuki M, Izawa M. In vitro and in vivo antibacterial activity of doripenem [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 32–46
Clinical and Laboratory Standards Institute. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard - seventh edition. M11-A7 Vol 27 No. 2. Wayne (PA): Clinical and Laboratory Standards Institute, 2008 Jan
Mushtaq S, Warner M, Ge Y, et al. In vitro interactions of doripenem with other antibacterials [abstract no. E-307]. 45th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 2005 Dec 16–19; Washington, DC
Foleno B, Wira E, Bush K, et al. Ceftobiprole in-vitro interactions with doripenem, levofloxacin, or colistin against Pseudomonas aeruginosa, Acinetobacter baumannii, and fluoroquinolone-resistant Escherichia coli [abstract no. 286]. 44th Annual Infectious Diseases Society of America; 2006 Oct 12–15; Toronto (ON), 99
Kobayashi Y. Study of the synergism between carbapenems and vancomycin or teicoplanin against MRSA, focusing on S-4661, a carbapenem newly developed in Japan. J Infect Chemother 2005 Oct; 11(5): 259–61
Ishii Y, Sugihara S, Tateda K, et al. In vitro synergistic effects of combinations of CP3242, as a novel metallo-β-lactamase inhibitor, and carbapenems against carbapenemase producing organisms [abstract no. F1-331]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Mushtaq S, Warner M, Kaniga K, et al. Bactericidal activity of doripenem vs. Pseudomonas aeruginosa [abstract no. F-1162 plus oral presentation]. 45th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 2005 Dec 16–19; Washington, DC, 187
Totsuka K, Kikuchi K. In vitro postantibiotic effect and in vivo antimicrobial activity of doripenem, a new carbapenem [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 52–6
Nishio H, Komatsu M, Shibata N, et al. Metallo-β-lactamase-producing Gram-negative bacilli: laboratory-based surveillance in cooperation with 13 clinical laboratories in the Kinki region of Japan. J Clin Microbiol 2004 Nov; 42(11): 5256–63
Deshpande LM, Jones RN, Fritsche TR, et al. Occurrence and characterization of carbapenemase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program (2000–2004). Microb Drug Resist 2006; 12(4): 223–30
Okamoto K, Gotoh N, Nishino T. Alterations of susceptibility of Pseudomonas aeruginosa by overproduction of multidrug efflux systems, MexAB-OprM, MexCD-OprJ, and MexXY/OprM to carbapenems: substrate specificities of the efflux systems. J Infect Chemother 2002 Dec; 8(4): 371–3
Li XZ, Nikaido H. Efflux-mediated drug resistance in bacteria. Drugs 2004; 64(2): 159–204
Mesaros N, Nordmann P, Plésiat P, et al. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect 2007 Jan; 13: 560–78
Pumbwe L, Ueda O, Yoshimura F, et al. Bacteroides fragilis BmeABC efflux systems additively confer intrinsic antimicrobial resistance. J Antimicrob Chemother 2006; 58(1): 37–46
Pumbwe L, Glass D, Wexler HM. Efflux pump overexpression in multiple-antibiotic-resistant mutants of Bacteroides fragilis. Antimicrob Agents Chemother2006 Sep; 50(9): 3150–3
Sakyo S, Tomita H, Tanimoto K, et al. Potency of carbapenems for the prevention of carbapenem-resistant mutants of Pseudomonas aeruginosa: the high potency of a new carbapenem doripenem. J Antibiot (Tokyo) 2006 Apr; 59(4): 220–8
Kim SY, Park YJ, Yu JK, et al. Prevalence and mechanisms of decreased susceptibility to carbapenems in Klebsiella pneumoniae isolates. Diagn Microbiol Infect Dis 2007 Jan; 57(1): 85–91
Morrow BJ, Andrew T, Queenan A, et al. P. aeruginosa DNA microarray analysis reveals differential induction of ampC and efflux by imipenem and doripenem [abstract no. C1-0037 plus poster]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA), 66
Queenan AM, Shang W, Flamm RK, et al. Effect of doripenem on AmpC induction in Enterobacteriaceae and Pseudomonas aeruginosa [abstract no. C1-0040 plus poster]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA), 66–7
Ramsey DM, Wozniak DJ. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol Microbiol 2005 Apr; 56(2): 309–22
Bagge N, Schuster M, Hentzer M, et al. Pseudomonas aeruginosa biofilms exposed to imipenem exhibit changes in global gene expression and β-lactamase and alginate production. Antimicrob Agents Chemother 2004 Apr; 48(4): 1175–87
Andrew T, Morrow B, Bush K, et al. Pseudomonas aeruginosa DNA microarray analysis reveals differential induction of alginate and repression of flagellar gene expression by imipenem and doripenem [abstract no. A097 plus poster]. 107th General Meeting of the American Society for Microbiology; 2007 May 21–25; Toronto (ON)
Huynh HK, Biedenbach DJ, Jones RN. Delayed resistance selection for doripenem when passaging Pseudomonas aeruginosa isolates with doripenem plus an aminoglycoside. Diagn Microbiol Infect Dis 2006 Jul; 55(3): 241–3
Hilliard JJ, Melton J, Zhang W, et al. In vivo activity of doripenem against systemic E. coli infections in the mouse [abstract no. 435]. 45th Annual Infectious Diseases Society of America; 2007 Oct 4–7; San Diego (CA), 139
Hilliard JJ, Melton J, Zhang W. In vivo activity of doripenem, imipenem, meropenem, and ceftazidime against systemic pseudomonal infections in the mouse [abstract no. 434]. 45th Annual Infectious Diseases Society of America; 2007 Oct 4–7; San Diego (CA), 139
Sato T, Tsuji M, Okazaki K, et al. Therapeutic efficacy of doripenem, a novel parenteral carbapenem antibiotic, against experimental infection in mice and rats [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 71–9
Katsube T, Yamano Y, Yano Y. Pharmacokinetic-pharmacodynamic modeling and simulation for in vivo bactericidal effect in murine infection model. J Pharm Sci 2008 Apr; 97(4): 1606–14
Hilliard J, Melton J, Zhang W, et al. In vivo anti-pseudomonal activity of doripenem [abstract no. 199]. 44th Annual Infectious Diseases Society of America; 2006 Oct 12–15; Toronto (ON), 81
Fernandez J, Zhang W,. In vivo activity of doripenem in a pseudomonal murine skin infection model [abstract no. 196]. 44th Annual Infectious Diseases Society of America; 2006 Oct 12–15; Toronto (ON)
Horiuchi M, Kimura M, Tokumura M, et al. Absence of convulsive liability of doripenem, a new carbapenem antibiotic, in comparison with β-lactam antibiotics. Toxicology 2006 May 1; 222(1–2): 114–24
Floren L, Wikler M, Kilfoil T, et al. A phase 1 open-label controlled study to evaluate the safety, tolerability, and pharmacokinetics of doripenem administered intravenously to subjects with renal impairment [abstract no. A-17 plus poster]. 44th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2004 Oct 30–Nov 2; Washington DC, 4
Nakashima M, Sesoko S, Oguma T. Phase I study of doripenem, a new carbapenem antibiotic for injection, in elderly volunteers [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 124–9
Nakashima M, Oguma T. Phase I study of doripenem, a new carbapenem antibiotic for injection in healthy volunteers [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 104–23
Shiba K, Nakashima M. Effect of probenecid on pharmacokinetics of doripenem in healthy male volunteers [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 136–42
Uehara S, Murao W, Seno Y, et al. Pharmacokinetics of doripenem in patients with renal dysfunction [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 130–5
Thye DA, Kilfoil T, Leighton A, et al. Doripenem: a phase 1 study to evaluate safety, tolerability and pharmacokinetics in a Western healthy volunteer population [abstract no. A-21 plus poster]. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2003 Sep 14–17; Chicago (IL)
Ikawa K, Morikawa N, Urakawa N, et al. Peritoneal penetration of doripenem after intravenous administration in abdominal-surgery patients. J Antimicrob Chemother 2007 Dec 1; 60(6): 1395–7
Tanimura H, Aikawa N, Sumiyama Y, et al. Pharmacokinetic profiles and clinical efficacy of doripenem in surgical infection [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 260–72
Okada H, Yasuda J, Hirabayashi K, et al. Basic and clinical studies on doripenem in obstetrics and gynecology [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 273–85
Baba S, Suzuki K, Miyamoto N. A study of distribution of doripenem in otolaryngologic tissues and clinical relevance in otolaryngologic infection [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 293–302
Sasaki J, Kaneko A. Clinical studies on doripenem of dentistry and oral surgery [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 323–31
Ooishi M, Miyanaga Y, Ohno S, et al. Study of doripenem distribution in ocular tissue and its clinical relevance in ophthalmological infection [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 313–22
Arata J, Watanabe S, Miyachi Y, et al. Laboratory and clinical evaluation of doripenem in deep-seated skin infection [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 303–12
Nakajima Y, Mizobuchi M, Nakamura M, et al. Mechanism of the drug interaction between valproic acid and carbapenem antibiotics in monkeys and rats. Drug Metab Dispos 2004 Dec; 32(12): 1383–91
Mori H, Takahashi K, Mizutani T. Interaction between valproic acid and carbapenem antibiotics. Drug Metab Rev 2007; 39(4): 647–57
Van Wart S, Bhavnani SM, Phillips L, et al. Population pharmacokinetics of doripenem [abstract no. A-18 plus poster]. 44th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2004 Oct 30–Nov 2; Washington DC, 4
Johnson & Johnson Pharmaceutical Research and Development LLC Raritan (NJ). Doribax (doripenem) injection new drug application 22–106. Clinical pharmacology and biopharmaceutics review(s) 2007 Oct 12 [online]. Available from URL: http://www.fda.gov.cder/foi/nda/2007/022106TOC.htm [Accessed 2008 May 19]
Hori T, Nakano M, Kimura Y, et al. Pharmacokinetics and tissue penetration of a new carbapenem, doripenem, intravenously administered to laboratory animals. In Vivo 2006 Jan–Feb; 20(1): 91–6
Mouton JW, Touw DJ, Horrevorts AM, et al. Comparative pharmacokinetics of the carbapenems: clinical implications. Clin Pharmacokinet 2000 Sep; 39: 185–201
Bhavnani SM, Hammel JP, Cirincione BB, et al. Use of pharmacokinetic-pharmacodynamic target attainment analyses to support phase 2 and 3 dosing strategies for doripenem. Antimicrob Agents Chemother 2005 Sep; 49(9): 3944–7
Andes DR, Kiem S, Craig WA. In vivo pharmacodynamic activity of a new carbapenem, doripenem (DOR), against multiple bacteria in a murine thigh infection model [abstract no. A-308]. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2003 Sep 14–17; Chicago (IL), 10
Andes DR, Craig WA, Bhavnani SM, et al. PK-PD evaluation of doripenem (DOR) against extended spectrum β-lactamase (ESBL) producing Enterobacteriaceae [abstract no. 182]. 41st Annual Meeting of the Infectious Diseases Society of America; 2003 Oct 9–12; San Diego (CA), 59
Ambrose PG, Phillips L, Bhavnani SM, et al. Use of pharmacokinetics-pharmacodynamics (PK-PD) as decision support for phase 3 dose justification for doripenem [abstract no. A-140]. 44th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2004 Oct 30–Nov 2; Washington DC, 12
Kuroda N, Munekage T, Yamano Y. Bactericidal activity of doripenem using in vitro pharmacodynamic models [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 96–103
Kim A, Banevicius MA, Nicolau DP. In vivo pharmacodynamic profiling of doripenem human simulated exposures against Pseudomonas aeruginosa. Antimicrob Agents Chemother. Epub 2008 May 5
Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008 (published erratum appears in Crit Care Med 2008 Apr; 36(4): 1394–6). Crit Care Med 2008 Jan; 36(1): 296–327
Réa-Neto A, Niederman M, Lobo SM, et al. Efficacy and safety of doripenem versus piperacillin/tazobactam in nosocomial pneumonia: a randomized, open-label, multicenter study. Curr Med Res Opin 2008 Jun 11; 24(7): 2113–26
Saito A, Watanabe A, Nakata K, et al. Comparative study of doripenem and meropenem in respiratory infections. Phase III double-blind comparative study [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 185–204
Saito A, Watanabe A, Odagiri S, et al. A dose-finding study on doripenem in chronic respiratory tract infection [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 169–84
Chastre J, Wunderink R, Prokocimer P, et al. Efficacy and safety of intravenous infusion of doripenem versus imipenem in ventilator-associated pneumonia: a multicenter, randomized study. Crit Care Med 2008 Apr; 36(4): 1089–96
Kollef MH, Rello J, Prokocimer P, et al. Efficacy of doripenem in nosocomial pneumonia: non-ventilator-associated and ventilator-associated pneumonia [abstract]. Chest 2007 Oct; 132(4 Suppl.): 498–9s
Lucasti C, Jasovich A, Umeh O, et al. Efficacy and tolerability of IV doripenem versus meropenem in adults with complicated intra-abdominal infection: a phase III, prospective, multicenter, randomized, double-blind, noninferiority study. Clin Ther 2008 May; 30(5): 868–83
Malafaia O, Umeh O, Jiang J. Doripenem versus meropenem for the treatment of complicated intra-abdominal infections [abstract no. L-1564b plus poster]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA)
Solomkin JS, Umeh O, Jiang J, et al. Doripenem vs. meropenem with an option for oral step-down therapy in the treatment of complicated intra-abdominal infections [abstract no. L-487 plus poster]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Naber K, Redman R, Kotey P, et al. Intravenous therapy with doripenem versus levofloxacin with an option for oral step-down therapy in the treatment of complicated urinary tract infections and pyelonephritis [abstract no. 833 plus poster]. 17th European Congress of Clinical Microbiology and Infectious Diseases and the 25th International Congress of Chemotherapy; 2007 Mar 31–Apr 3; Munich
Kamidono S, Arakawa S, Hirose T, et al. Double-blind, controlled study to evaluate safety and efficacy of doripenem and meropenem in patients with complicated urinary tract infection [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 244–59
Saito A, Shimada J, Shiba K, et al. A study of doripenem efficacy and safety in internal medicine [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 157–68
Shimada J, Yamaguchi K, Shiba K, et al. Early phase II study of doripenem, a new carbapenem antibiotic for injection [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 143–56
Kamidono S, Arakawa S, Kumon H, et al. A dose-finding study of doripenem in patients with complicated urinary tract infection [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 230–43
Kamidono S. Late phase II study of doripenem in urological infections [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 216–29
Saito A, Kamidono S, Yokoyama T, et al. Study of safety and efficacy of doripenem in patients with sepsis and infective endocarditis [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 332–40
Saito A, Watanabe A, Itabashi S, et al. Therapeutic exploratory study of the efficacy and safety of doripenem in patients with hospital-acquired pneumonia [in Japanese]. Jpn J Chemother 2005; 53 Suppl. 1: 205–15
Merchant S, Gast C, Nathwani D, et al. Hospital resource utilization with doripenem versus imipenem in the treatment of ventilator-associated pneumonia. Clin Ther 2008 Apr; 30(4): 717–33
Psathas PA, Kuzmission A, Ikeda K, et al. Stability of doripenem for injection (500 mg) in representative infusion fluids and containers [abstract no. 57E]. 2007 ASHP meeting; 2007 Jun 27; San Francisco (CA)
Joseph J, Rodvold KA. The role of carbapenems in the treatment of severe nosocomial respiratory tract infections. Expert Opin Pharmacother 2008 Mar; 9(4): 561–75
Helfand MS, Bonomo RA. Current challenges in antimicrobial chemotherapy: the impact of extended-spectrum β-lactamases and metallo-β-lactamases on the treatment of resistant Gramnegative pathogens. Curr Opin Pharmacol 2005 Oct; 5(5): 452–8
Kollef MH. Appropriate empiric antimicrobial therapy of nosocomial pneumonia: the role of the carbapenems. Respir Care 2004 Dec; 49(12): 1530–41
Johnson & Johnson Pharmaceutical Research and Development LLC, Raritan (NJ). FDA extends review timeline for additional indication for antibiotic DORIBAX™ 2008 Mar 6 [media release]. Available from URL: http://www.jnj.com/news [Accessed 2008 Apr 7]
AstraZeneca Pharmaceuticals LP, Wilmington (DE). Merrem® I.V. (meropenem for injection). US prescribing information [online]. Available from URL: http://www.astrazenecaus.com/pi/MerremIV.pdf [Accessed 2008 May 20]
Merck and Co Inc., Whitehouse Station (NJ). Primaxin® I.V. (imipenem and cilastin for injection). US prescribing information 2007 Dec [online]. Available from URL: http://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf [Accessed 2008 May 20]
Baldwin CM, Lyseng-Williamson K, Keam SJ. Meropenem: a review of its use in the treatment of serious bacterial infections. Drugs 2008; 68(6): 803–41
Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005 Nov 15; 41(10): 1373–406
Solomkin JS, Mazuski JE, Baron EJ, et al. Guidelines for the selection of anti-infective agents for complicated intra-abdominal infections. Clin Infect Dis 2003 Oct 15; 37(8): 997–1005
Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. ERS Task Force in collaboration with ESCMID. Eur Respir J 2005 Dec; 26(6): 1138–80
Wilkinson M, Woodhead MA. Guidelines for community-acquired pneumonia in the ICU. Curr Opin Crit Care 2004 Feb; 10(1): 59–64
Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. American Thoracic Society. Am J Respir Crit Care Med 2005 Feb 15; 171(4): 388–416
Naber KG, Bishop MC, Bjerklund-Johansen TE, et al. Guidelines on the management of urinary and male genital tract infections. European Association of Urology [online]. Available from URL: http://www.uroweb.org/fileadmin/user_upload/Guidelines/15%20Male%20UTI.pdf [Accessed 2008 Mar 11]
Gilbert DN, Moellering RC, Eliopoulos GM, et al, editors. Sanford guide to antimicrobial therapy 2008. 38th ed. Sperryville (VA): Antimicrobial Therapy Inc, 2008 Apr 15
Tanaka A, Takada T, Kawarada Y, et al. Antimicrobial therapy for acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg 2007; 14(1): 59–67
Miyashita N, Matsushima T, Oka M. The JRS guidelines for the management of community-acquired pneumonia in adults: an update and new recommendations. Intern Med 2006; 45(7): 419–28
Paterson DL, Rossi F, Baquero F, et al. In vitro susceptibilities of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: the 2003 Study for Monitoring Antimicrobial Resistance Trends (SMART). J Antimicrob Chemother 2005 Jun; 55(6): 965–73
Rice LB. Challenges in identifying new antimicrobial agents effective for treating infections with Acinetobacter baumannii and Pseudomonas aeruginosa. Clin Infect Dis 2006 Sep 1; 43 Suppl. 2: S100–5
Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 2007; 67(3): 351–68
Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med 2008 Mar 20; 358(12): 1271–81
Gilad J, Carmeli Y. Treatment options for multidrug-resistant Acinetobacter species. Drugs 2008; 68(2): 165–89
Giamarellou H, Kanellakopoulou K. Current therapies for Pseudomonas aeruginosa. Crit Care Clin 2008 Apr; 24(2): 261–78
Felmingham D. Comparative antimicrobial susceptibility of respiratory tract pathogens. Chemotherapy 2004; 50 Suppl. 1: 3–10
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: Y. Ike, Gunma University Graduate School of Medicine, Maebashi, Japan; T. Matsumoto, Department of Urology, University of Occupational and Environmental Health, Kitakyushu, Japan; G. Poulakou, 4th Department of Internal Medicine, Athens Medical School, Attikon University General Hospital, Athens, Greece; J.S. Solomkin, Department of Surgery, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA; F. Van Bambeke, Unite de Pharmacologie Cellulaire et Moleculaire, Universite Catholique de Louvain, Brussels, Belgium; G.G. Zhanel, Medical Microbiology Health Sciences Centre, University of Manitoba, Winnipeg, Manitoba, Canada.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘doripenem’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Wolters Kluwer Health | Adis). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search term was ‘doripenem’. Searches were last updated 4 August 2008.
Selection: Studies in patients with bacterial infections who received doripenem. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Doripenem, complicated intra-abdominal infections, complicated skin and skin structure infections, complicated urinary tract infections, nosocomial pneumonia, ventilator-associated pneumonia, gynaecological and obstetric infections, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Keam, S.J. Doripenem. Drugs 68, 2021–2057 (2008). https://doi.org/10.2165/00003495-200868140-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200868140-00007