Abstract
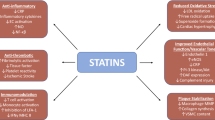
Molecular differences among the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) give rise to some important differences in their properties, including their anti-atherogenic and anti-inflammatory actions (among the so-called pleiotropic effects) - differences that may help to account for variation in clinical efficacy and safety among the available drugs of this class.
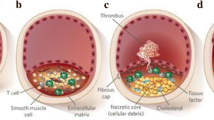
The question of whether the clinical benefit of statins such as atorvastatin in reducing cardiovascular events in individuals with elevated cholesterol levels results from direct anti-atherogenic effects in addition to cholesterol-lowering-dependent effects cannot be conclusively answered at present. However, the available evidence suggests that these actions should be considered further, especially in some clinical situations such as acute coronary syndrome where an anti-inflammatory effect could conceivably have a greater role. In the case of atorvastatin, various direct anti-atherogenic effects have been demonstrated. These effects include modification of endothelial dysfunction, inflammatory processes and lipid oxidation, and a possible direct effect on the composition of atheromatous plaques, which together may have a positive influence on the development of atherosclerosis and its subsequent progression (e.g. on the reduction of carotid intima media thickness and regression of atheromas noted in studies with intensive atorvastatin therapy [80 mg/day]). In terms of its effects on endothelial function, improvements in flow-mediated endothelium-dependent vasodilatation have been observed as early as 2 weeks after starting atorvastatin treatment. This effect does not appear to be quantitatively correlated with lowering of low-density lipoprotein-cholesterol (LDL-C) as greater improvements in endothelial function versus ezetimibe/simvastatin have been noted with atorvastatin despite comparable reductions in LDL-C. Atorvastatin has also been shown to reduce levels of the inflammatory marker C-reactive protein; in comparative studies, this effect proved to be superior to that of simvastatin or pravastatin and equivalent to that of rosuvastatin. In other studies, atorvastatin has been found to inhibit the in vitro oxidation of LDL — an effect that appears to be due mainly its active hydroxy metabolite — and to reduce various oxidative stress markers in hypercholesterolaemic patients. In addition, there is evidence that atorvastatin is able to modify the composition of atherosclerotic plaques and their inflammatory status via a series of effects, mostly involving tissue factors.
Similar content being viewed by others
References
Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295(13): 1556–65
Mason RP, Walter MF, Day CA, et al. Intermolecular differences of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors contribute to distinct pharmacologic and pleiotropic actions. Am J Cardiol 2005; 96(5A): 11–23F
Mason RP. Molecular basis of differences among statins and a comparison with antioxidant vitamins. Am J Cardiol 2006; 98(11A): 34–41P
Christopher-Stine L. Statin myopathy: an update. Curr Opin Rheumatol 2006; 18(6): 647–53
Baker SK. Molecular clues into the pathogenesis of statin-mediated muscle toxicity. Muscle Nerve 2005; 31(5): 572–80
Davidson MH. Clinical significance of statin pleiotropic effects: hypotheses versus evidence. Circulation 2005; 111(18): 2280–1
Verges B. Do the pleiotropic effects of statins have a clinical significance? [in French]. Arch Mal Coeur Vaiss 2004; 97(12): 1231–5
Robinson JG, Smith B, Maheshwari N, et al. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol 2005; 46(10): 1855–62
O’Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999; 340(1): 14–22
Smilde TJ, van Wissen S, Wollersheim H, et al. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet 2001; 357(9256): 577–81
Taylor AJ, Kent SM, Flaherty PJ, et al. ARBITER, Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation 2002; 106(16): 2055–60
Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004; 291(9): 1071–80
Blumenthal RS, Kapur NK. Can a potent statin actually regress coronary atherosclerosis? JAMA 2006; 295(13): 1583–4
Malhotra HS, Goa KL. Atorvastatin: an updated review of its pharmacological properties and use in dyslipidaemia. Drugs 2001; 61(12): 1835–81
Simons LA, Sullivan D, Simons J, et al. Effects of atorvastatin monotherapy and simvastatin plus cholestyramine on arterial endothelial function in patients with severe primary hypercholesterolaemia. Atherosclerosis 1998; 137(1): 197–203
Marchesi S, Lupattelli G, Siepi D, et al. Short-term atorvastatin treatment improves endothelial function in hypercholesterolemic women. J Cardiovasc Pharmacol 2000; 36(5): 617–21
Wagner AH, Kohler T, Ruckschloss U, et al. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial Superoxide anion formation. Arterioscler Thromb Vasc Biol 2000; 20(1): 61–9
Feron O, Dessy C, Desager JP, et al. Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation 2001; 103(1): 113–8
Ortego M, Bustos C, Hernandez-Presa MA, et al. Atorvastatin reduces NF-kappaB activation and chemokine expression in vascular smooth muscle cells and mononuclear cells. Atherosclerosis 1999; 147(2): 253–61
Bustos C, Hernandez-Presa MA, Ortego M, et al. HMG-CoA reductase inhibition by atorvastatin reduces neointimal inflammation in a rabbit model of atherosclerosis. J Am Coll Cardiol 1998; 32(7): 2057–64
Bellosta S, Ferri N, Arnaboldi L, et al. Pleiotropic effects of statins in atherosclerosis and diabetes. Diabetes Care 2000; 23 Suppl. 2: B72–88
Axel DI, Riessen R, Runge H, et al. Effect of the new HMG-CoA reductase inhibitor atorvastatin on human vascular cell growth in mono- and co-cultures in comparison to lovastatin [abstract]. Eur Heart J 1997; 18 Suppl. A: 370
Tannous M, Cheung R, Vignini A, et al. Atorvastatin increases ecNOS levels in human platelets of hyperlipidemic subjects. Thromb Haemost 1999; 82(5): 1390–4
Fan B, Tomlinson B, Critchley JAJH. Effects of atorvastatin on platelet aggregation in whole blood [abstract]. Atherosclerosis 1999; 35 Suppl. 1: 35
Porreca E, Di Febbo C, Amore C, et al. Effect of lipid-lowering treatment on factor VII profile in hyperlipidemic patients. Thromb Haemost 2000; 84(5): 789–93
Atalar E, Acil T, Aytemir K, et al. Effects of atorvastatin treatment on global fibrinolytic capacity, apoptosis, and leukocyte activation in patients with coronary artery disease [abstract]. J Am Coll Cardiol 2001; 37 Suppl. A: 267
Aviram M, Rosenblat M, Bisgaier CL, et al. Atorvastatin and gemfibrozil metabolites, but not the parent drugs, are potent antioxidants against lipoprotein oxidation. Atherosclerosis 1998; 138(2): 271–80
Bocan TM, Mueller SB, Brown EQ, et al. HMG-CoA reductase and ACAT inhibitors act synergistically to lower plasma cholesterol and limit atherosclerotic lesion development in the cholesterol-fed rabbit. Atherosclerosis 1998; 139(1): 21–30
Shishehbor MH, Brennan ML, Aviles RJ, et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation 2003; 108(4): 426–31
Jialal I, Stein D, Balis D, et al. Effect of HMG-CoA reductase inhibitor therapy on C-reactive protein levels [abstract]. Circulation 2000; 102 Suppl.: 11–833
Asberg A, Hartmann A, Fjeldsa E, et al. Atorvastatin improves endothelial function in renal-transplant recipients. Nephrol Dial Transplant 2001; 16(9): 1920–4
Mullen MJ, Wright D, Donald AE, et al. Atorvastatin but not L-arginine improves endothelial function in type I diabetes mellitus: a double-blind study. J Am Coll Cardiol 2000; 36(2): 410–6
Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial Superoxide anion production. J Clin Invest 1993; 91(6): 2546–51
Fichtischerer S, Schmidt-Lucke C, Bojunga S, et al. Differential effects of short-term lipid lowering with ezetimibe and statins on endothelial function in patients with CAD: clinical evidence for ‘pleiotropic’ functions of statin therapy. Eur Heart J 2006; 27(10): 1182–90
Leung WH, Lau CP, Wong CK. Beneficial effect of cholesterol-lowering therapy on coronary endothelium-dependent relaxation in hypercholesterolaemic patients. Lancet 1993; 341(8859): 1496–500
Kinlay S, Timms T, Clark M, et al. Comparison of effect of intensive lipid lowering with atorvastatin to less intensive lowering with lovastatin on C-reactive protein in patients with stable angina pectoris and inducible myocardial ischemia. Am J Cardiol 2002; 89(10): 1205–7
van Wissen S, Trip MD, Smilde TJ, et al. Differential hs-CRP reduction in patients with familial hypercholesterolemia treated with aggressive or conventional statin therapy. Atherosclerosis 2002; 165(2): 361–6
Wiklund O, Mattsson-Hulten L, Hurt-Camejo E, et al. Effects of simvastatin and atorvastatin on inflammation markers in plasma. J Intern Med 2002; 251(4): 338–47
Stalenhoef AF, Ballantyne CM, Sarti C, et al. A comparative study with rosuvastatin in subjects with metabolic syndrome: results of the COMETS study. Eur Heart J 2005; 26(24): 2664–72
Ballantyne CM, Abate N, Yuan Z, et al. Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study. Am Heart J 2005; 149(3): 464–73
Karaca I, Ilkay E, Akbulut M, et al. Atorvastatin affects C-reactive protein levels in patients with coronary artery disease. Curr Med Res Opin 2003; 19(3): 187–91
Riesen WF, Engler H, Risch M, et al. Short-term effects of atorvastatin on C-reactive protein. Eur Heart J 2002; 23(10): 794–9
Otto C, Geiss HC, Empen K, et al. Long-term reduction of C-reactive protein concentration by regular LDL apheresis. Atherosclerosis 2004; 174(1): 151–6
Devaraj S, Autret B, Jialal I. Effects of colesevelam hydrochloride (WelChol) on biomarkers of inflammation in patients with mild hypercholesterolemia. Am J Cardiol 2006; 98(5): 641–3
Liao JK. Beyond lipid lowering: the role of statins in vascular protection. Int J Cardiol 2002; 86(1): 5–18
Walter MF, Jacob RF, Weng Y, et al. Active hydroxy metabolite of atorvastatin increases resistance of human low-density lipoproteins to oxidative modification [abstract]. 53rd Annual Scientific Session, American College of Cardiology; 2004 Mar 7–10; New Orleans (LA)
Mason RP, Walter MF, Day CA, et al. Active metabolite of atorvastatin inhibits membrane cholesterol domain formation by an antioxidant mechanism. J Biol Chem 2006; 281(14): 9337–45
Annovazzi A, Bonanno E, Area M, et al. 99mTc-Interleukin-2 scintigraphy for the in vivo imaging of vulnerable atherosclerotic plaques. Eur J Nucl Med Mol Imaging 2006; 33(2): 117–26
Cortellaro M, Cofrancesco E, Arbustini E, et al. Atorvastatin and thrombogenicity of the carotid atherosclerotic plaque: the ATROCAP study. Thromb Haemost 2002; 88(1): 41–7
Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 2005; 45: 89–118
The Lipid Research Clinics Coronary Primary Prevention Trial. The Lipid Research Clinics Coronary Primary Prevention Trial results, II: the relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984; 251(3): 365–74
Buchwald H, Varco RL, Matts JP, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia. Report of the Program on the Surgical Control of the Hyperlipidemias (POSCH). N Engl J Med 1990; 323(14): 946–55
Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995; 333(20): 1301–7
Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996; 335(14): 1001–9
The Scandinavian Simvastatin Survival Study (4S). Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344(8934): 1383–9
Nissen SE. High-dose statins in acute coronary syndromes: not just lipid levels. JAMA 2004; 292(11): 1365–7
Briel M, Studer M, Glass TR, et al. Effects of statins on stroke prevention in patients with and without coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med 2004; 117(8): 596–606
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rubba, P. Effects of Atorvastatin on the Different Phases of Atherogenesis. Drugs 67 (Suppl 1), 17–27 (2007). https://doi.org/10.2165/00003495-200767001-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200767001-00003