Summary
Abstract
Inhaled human insulin (Exubera® (insulin human [rDNA origin]) Inhalation Powder) has recently been approved in the European Union and the US for preprandial use in adult patients with diabetes mellitus. This formulation of insulin has a more rapid onset, but similar duration, of glucose-lowering activity compared with subcutaneously administered regular human insulin.
Preprandial inhaled human insulin provided glycaemic control that was comparable to preprandial subcutaneous regular insulin when added to long- or intermediate-acting subcutaneous basal insulin in patients with type 1 diabetes mellitus. Inhaled human insulin is also effective when administered alone, when combined with oral antihyperglycaemic therapy, or when combined with basal subcutaneous insulin in patients with type 2 diabetes mellitus. Comparable rates of hypoglycaemia occurred in patients treated with inhaled human insulin and in those treated with subcutaneous regular human insulin. Patients treated with inhaled human insulin demonstrated a greater decline in pulmonary function (forced expiratory volume in 1 second [FEV1], carbon monoxide diffusing capacity [DLco]) than patients treated with comparator antihyperglycaemic agents; the mean difference between the treatment groups that favoured the comparators was noted within the first several weeks of treatment, and did not change over a 2-year treatment period. This agent has also been associated with significant improvements in some quality-of-life and treatment satisfaction scores, especially when compared with subcutaneous mealtime insulin regimens. Inhaled human insulin is an effective and well tolerated formulation suitable for preprandial use in combination with basal subcutaneous insulin in patients with type 1 diabetes. It is also well tolerated and effective in patients with type 2 diabetes when administered alone, when combined with oral antihyperglycaemic therapy, or when combined with basal subcutaneous insulin.
Pharmacological Properties
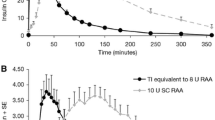
In healthy volunteers and in patients with diabetes, plasma concentrations of insulin increase more rapidly after administration of inhaled human insulin than after a subcutaneous injection of regular human insulin. With inhaled human insulin, the maximal antihyperglycaemic effect occurred after approximately 2 hours, and the duration of action was around 6 hours. Inhaled human insulin had a significantly faster onset of glucose-lowering effect than subcutaneous regular insulin (p < 0.001). Times to maximal glucose-lowering effect were comparable for inhaled human insulin and the rapid-acting insulin lispro, but shorter for inhaled than for regular insulin (p < 0.01). The duration of glucose-lowering effect of inhaled human insulin was comparable to that of subcutaneously administered regular insulin, but longer than that of subcutaneously administered insulin lispro (p < 0.01). Intra-individual reproducibility of glycaemic response is at least as good as that with subcutaneous regular insulin. Cigarette smoking substantially increases systemic exposure to inhaled human insulin; inhaled human insulin is contra-indicated in patients who smoke or who have smoked 6 months prior to starting therapy.
Therapeutic Efficacy
In 24-week phase III trials in patients with type 1 diabetes treated with basal insulin, the reduction in glycosylated haemoglobin (HbA1c) values (0.1–0.5 percentage points from baseline) in recipients of preprandial inhaled human insulin was comparable to that with subcutaneous regular insulin. The percentage of patients achieving HbA1c values of <7% was comparable between the two treatment groups.
In patients with type 2 diabetes inadequately controlled with diet and exercise alone, HbA1c values below 8% were achieved in 83% of patients treated with inhaled human insulin. Mean HbA1c values were reduced by 2.3% over the 24 weeks of treatment. Mean HbA1c reductions were maintained to a greater extent with inhaled human insulin than with oral antihyperglycaemic treatment in a 104-week extension study in type 2 diabetic patients not responding to oral antihyperglycaemic monotherapy. In patients with type 2 diabetes poorly controlled by combination oral antihyperglycaemic therapy, reductions in HbA1c levels from baseline were significantly greater with inhaled human insulin (as monotherapy or in combination with oral antihyperglycaemic agents) than with oral antihyperglycaemic agents alone. Use of inhaled human insulin before meals with basal ultralente insulin at bedtime was similar to treatment with mixed subcutaneous neutral protamine Hagedorn (NPH)/regular insulin in controlling HbA1c in patients with type 2 diabetes previously treated with subcutaneous insulin.
Inhaled human insulin was associated with significant improvements in some quality-of-life and treatment satisfaction scores in patients with diabetes, especially when compared with subcutaneous mealtime insulin regimens.
Tolerability
Rates of hypoglycaemia were comparable in recipients of inhaled human insulin or subcutaneous regular insulin in randomised clinical trials. Treatment of diabetic patients with inhaled human insulin was generally associated with increases in bodyweight (0.1–3.6kg); however, the increase was numerically less than that with subcutaneous regular insulin. Patients treated with inhaled human insulin demonstrated a greater decline in pulmonary function (FEV1 and DLco) than patients treated with comparator antihyperglycaemic agents. The mean difference between the treatment groups that favoured the comparators was noted within the first several weeks of treatment, and did not change over a 2-year treatment period. It is recommended that patients should have pulmonary function assessments prior to initiating inhaled insulin therapy and at periodic intervals thereafter. An increased incidence of cough, which occurs within seconds to minutes of inhalation, has been reported, but this has been of mild to moderate severity and has tended to decrease in frequency over time. Greater insulin antibody production with inhaled human insulin than with subcutaneous regular human insulin given at mealtimes has been reported in patients with diabetes, but this appears to have no clinical relevance in terms of glycaemic control or pulmonary function.








Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
International Diabetes Federation. Diabetes atlas: prevalence [online]. Available from URL: http://www.eatlas.idf.org/Prevalence [Accessed 2006 Feb 9]
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993 Sep 30; 329(14): 977–86
Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995 May; 28(2): 103–17
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000 Aug 12; 321(7258): 405–12
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998 Sep 12; 352(9131): 837–53
Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia 1996 Dec; 39(12): 1577–83
Zambanini A, Newson RB, Maisey M, et al. Injection related anxiety in insulin-treated diabetes. Diabetes Res Clin Pract 1999 Dec; 46(3): 239–46
Mollema ED, Snoek FJ, Heine RJ, et al. Phobia of self-injecting and self-testing in insulin-treated diabetes patients: opportunities for screening. Diabet Med 2001 Aug; 18(8): 671–4
Galloway JA, Spradlin CT, Nelson RL, et al. Factors influencing the absorption, serum insulin concentration, and blood glucose responses after injections of regular insulin and various insulin mixtures. Diabetes Care 1981 May–Jun 30; 4(3): 366–76
Binder C, Lauritzen T, Faber O, et al. Insulin pharmacokinetics. Diabetes Care 1984 Mar–1984 30; 7(2): 188–99
Harsch IA. Inhaled insulins: their potential in the treatment of diabetes mellitus. Treat Endocrinol 2005; 4(3): 131–8
Lenzer J. Inhaled insulin is approved in Europe and United States. BMJ 2006 Feb 11; 332(7537): 321
Patton JS, Bukar JG, Eldon MA. Clinical pharmacokinetics and pharmacodynamics of inhaled insulin. Clin Pharmacokinet 2004; 43(12): 781–801
Byron P. Prediction of drug residence times in regions of the human respiratory tract following aerosol inhalation. J Pharm Sci 1986; 75: 433–8
Rave K, Bott S, Heinemann L, et al. Time-action profile of inhaled insulin in comparison with subcutaneously injected insulin lispro and regular human insulin. Diabetes Care 2005 May; 28(5): 1077–82
Pfizer Inc. Exubera® (insulin human [rDNA origin]) inhalation powder: US package insert. 2006 Jan
Gelfand RA, Schwartz SL, Horton M, et al. Pharmacological reproducibility of inhaled human insulin pre-meal dosing in patients with type 2 diabetes mellitus. Diabetes 1998 May; 47 Suppl. 1: 99
Henry R, Mudaliar S, Fryburg D, et al. Within-subject variability of inhaled insulin (Exubera®) versus subcutaneous regular insulin in elderly obese patients with type 2 diabetes mellitus [abstract no. 488-P]. Diabetes 2003; 52 Suppl. 1: 105. Plus poster presented at the 63rd Annual Scientific Sessions of the American Diabetes Association, 2003 Jun 13–17, New Orleans (LA)
Becker RH, Sha S, Frick AD, et al. The effect of smoking cessation and subsequent resumption on absorption of inhaled insulin. Diabetes Care 2006 Feb; 29(2): 277–82
Sha S, Becker RHA, Willvise SA, et al. The effect of smoking cessation on the absorption of inhaled insulin (Exubera®) [abstract no. 538-P]. Diabetes 2002; 51 Suppl. 2: 133. Plus poster presented at the 61st Annual Scientific Sessions of the American Diabetes Association, 2001 Jun 22–26, Philadelphia (PA)
Skyler JS, Weinstock RS, Raskin P, et al. Use of inhaled insulin in a basal/bolus insulin regimen in type 1 diabetic subjects: a 6-month, randomized, comparative trial. Diabetes Care 2005 Jul; 28(7): 1630–5
DeFronzo RA, Bergenstal RM, Cefalu WT, et al. Efficacy of inhaled insulin in patients with type 2 diabetes not controlled with diet and exercise: a 12-week, randomized, comparative trial. Diabetes Care 2005 Aug; 28(8): 1922–8
Hollander PA, Blonde L, Rowe R, et al. Efficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patients with type 2 diabetes: results of a 6-month, randomized, comparative trial. Diabetes Care 2004 Oct; 27(10): 2356–62
Rosenstock J, Zinman B, Murphy LJ, et al. Inhaled insulin improves glycemic control when substituted for or added to oral combination therapy in type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2005 Oct 18; 143(8): 549–58
Skyler JS, Cefalu WT, Kourides IA, et al. Efficacy of inhaled human insulin in type 1 diabetes mellitus: a randomised proof-of-concept study. Lancet 2001 Feb 3; 357: 331–5
Cappelleri JC, Cefalu WT, Rosenstock J, et al. Treatment satisfaction in type 2 diabetes: a comparison between an inhaled insulin regimen and a subcutaneous insulin regimen. Clin Ther 2002 Apr; 24: 552–64
Weiss SR, Cheng SL, Kourides IA, et al. Inhaled insulin provides improved glycémie control in patients with type 2 diabetes mellitus inadequately controlled with oral agents: a randomized controlled trial. Arch Intern Med 2003 Oct 27; 163(19): 2277–82
Dumas R, England RD, Riese RJ, et al. Exubera® is well tolerated and achieves tight glycémie control in patients with type 1 diabetes. Diabetes 2005 Jun; 54: 87
Quattrin T, Belanger A, Bohannon NJV, et al. Efficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patients with type 1 diabetes: results of a 6-month, randomized, comparative trial. Diabetes Care 2004 Nov; 27(11): 2622–7
Barnett AH, Exubera® Phase III Study Group. Efficacy and one-year pulmonary safety of inhaled insulin (Exubera®) as adjunctive therapy with metformin or glibenclamide in type 2 diabetes patients poorly controlled on oral agent monotherapy [abstract no. 454-P]. Diabetes 2004; 53 Suppl. 2: 107. Plus poster presented at the 64th Annual Scientific Sessions of the American Diabetes Association; 4–8 Jun 2004; Orlando (FL)
Bergenstal RM. Achieving target HbA1c in studies with inhaled insulin in type 2 diabetes [abstract no. 866]. Diabetologia 2004 Aug; 47 Suppl. 1: 312
Dreyer M. Efficacy and two-year pulmonary safety of inhaled insulin as adjunctive therapy with metformin or glibenclamide in type 2 diabetes patients poorly controlled with oral monotherapy [abstract no. 114]. Diabetologia 2004 Aug; 47 Suppl. 1: 44–5
Simonson DC, Hayes JF, Turner RR, et al. Treatment satisfaction and preferences in type 2 diabetes: a randomized trial of oral agents vs. inhaled insulin [abstract no. 528-P]. Diabetes 2001; 50 Suppl. 2
Testa MA, Turner RR, Hayes JF, et al. Intensive therapy and patient satisfaction in type 1 diabetes: a randomized trial of injected vs. inhaled insulin [abstract no. 8]. Diabetologia 2001; 44 Suppl. 1: 4
Testa MA, Hayes JF, Turner RR, et al. Quality of life improvements in type 2 diabetes when Exubera® is added after failure on metformin monotheapy: an international randomized phase 3 trial [abstract no. 1831-P]. Diabetes 2004 Jun; 53 Suppl. 2: 437
Testa MA, Turner RR, Hayes JF, et al. An international trial of sulfonylurea plus either metformin or Exubera®: impact on quality of life and treatment satisfaction [abstract no. 9]. Diabetologia 2004 Aug; 47 Suppl. 2: 5–6
Freemantle N, Blonde L, Duhot D, et al. Availability of inhaled insulin promotes greater perceived acceptance of insulin therapy in patients with type 2 diabetes. Diabetes Care 2005 Feb; 28(2): 427–8
Skyler J, Exubera® Phase II Study Group. Sustained long-term efficacy and safety of inhaled insulin during 4 years of continuous therapy [abstract 486-P]. Diabetes 2004 Jun; 53 Suppl. 2: 115
Heise T, Bott S, Tusek C, et al. The effect of insulin antibodies on the metabolic action of inhaled and subcutaneous insulin: a prospective randomized pharmacodynamic study. Diabetes Care 2005 Sep; 28(9): 2161–9
Fineberg SE, Kawabata T, Finco-Kent D, et al. Antibody response to inhaled insulin in patients with type 1 or type 2 diabetes. An analysis of initial phase II and III inhaled insulin (Exubera) trials and a two-year extension trial. J Clin Endocrinol Metab 2005 Jun; 90(6): 3287–94
Dumas R, Krasner AS, England RD, et al. Immunologic response to Exubera® in patients with type 1 diabetes is not associated with functional evidence of airway sensitization. Diabetes 2005 Jun 1; 54 Suppl. 1: 108
Owens DR, Zinman B, Bolli G. Alternative routes of insulin delivery. Diabet Med 2003 Nov; 20(11): 886–98
American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005 Jan; 28 Suppl. 1: S4–36
Ristic S, Bates PC. Effects of rapid-acting insulin analogs on overall glycemic control in type 1 and type 2 diabetes mellitus. Diabetes Technol Ther 2003; 5(1): 57–66
National Institute for Health and Clinical Excellence. Clinical guidelines for type 2 diabetes: management of blood glucose [online]. Available from URL: http://www.nice.org.uk/pdf/NICE_full_blood_glucose.pdf [Accessed 2006 Feb 13]
National Institute for Health and Clinical Excellence. Type 1 diabetes: diagnosis and management of type 1 diabetes in children, young people and adults [online]. Available from URL: http://www.nice.org.uk [Accessed 2006 Feb 6]
American Association of Clinical Endocrinologists. The American Association of Clinical Endocrinologists medical guidelines for the management of diabetes mellitus: the AACE system of intensive diabetes self-management. 2002 update. Endocr Pract 2002; 8 Suppl. 1: S40–82
Hauber AB, Johnson FR, Sauriol L, et al. Risking health to avoid injections: preferences of Canadians with type 2 diabetes. Diabetes Care 2005 Sep; 28(9): 2243–5
Harris MI. Health care and health status and outcomes for patients with type 2 diabetes. Diabetes Care 2000 Jun; 23(6): 754–8
Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord 2002 Sep; 26 Suppl. 3: S18–24
Gale EAM. Two cheers for inhaled insulin. Lancet 2001 Feb 3; 357: 324–5
Sadri H, MacKeigan LD, Leiter LA, et al. Willingness to pay for inhaled insulin: a contingent valuation approach. Pharma-coeconomics 2005; 23(12): 1215–27
King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998 Sep; 21(9): 1414–31
Jönsson B. Revealing the cost of type II diabetes in Europe. Diabetologia 2002 Jul; 45(7): S5–12
Valente AXCN, Langer R, Stone HA, et al. Recent advances in the development of an inhaled insulin product. Biodrugs 2003; 17(1): 9–17
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: K. Capoccia, University of Washington, Seattle, Washington, USA; N. Freemantle, University of Birmingham, Birmingham, United Kingdom; I.A. Harsch, University of Erlangen, Erlangen, Germany; T. Heise, Profil Institut für Stoffwechselforschung, Neuss, Germany; T.K. Mandal, Xavier University of Louisiana, New Orleans, Louisiana, USA; D.R. Owens, Diabetes Research Unit, Llandough Hospital, Penarth, United Kingdom; T. Quattrin, State University of New York at Buffalo, Buffalo, New York, USA; H. Sadri, Department of Clinical Pharmacology, Health Outcomes and Pharmacoeconomics Research Centre (HOPE), Sunnybrook Hospital, Toronto, Ontario, Canada.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘inhaled insulin’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE search terms were ‘inhaled insulin’ or ‘insulin inhalation’. EMBASE search terms were ‘inhaled insulin’ or ‘insulin inhalation’. AdisBase search terms were ‘inhaled insulin’ or ‘insulin inhalation’. Searches were last updated 17 May 2006.
Selection: Studies in patients with type 1 or 2 diabetes mellitus who received inhaled insulin. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Inhaled human insulin, type 1 diabetes mellitus, type 2 diabetes mellitus, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Dunn, C., Curran, M.P. Inhaled Human Insulin (Exubera®). Drugs 66, 1013–1032 (2006). https://doi.org/10.2165/00003495-200666070-00019
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200666070-00019