Summary
Abstract
Darbepoetin alfa (Aranesp®) is an analogue of recombinant human erythropoietin (rHuEPO) produced using recombinant DNA technology. The high number of sialic acid moieties in darbepoetin alfa results in a prolonged half-life and enhanced in vivo biological activity compared with rHuEPO (as demonstrated in animal studies) and permits a reduction in the frequency of administration.
Subcutaneous darbepoetin alfa 2.25 μg/kg once weekly or 500μg once every 3 weeks (with a provision for dose adjustments) is an effective and well tolerated erythropoietic agent in anaemic patients with cancer receiving chemotherapy. In randomised, controlled clinical trials, the drug increased haemoglobin levels and reduced the need for blood transfusions in patients with various types of nonmyeloid malignancies and also ameliorated anaemia-related fatigue, thereby improving their health-related quality of life (HR-QOL) scores. The once-every-3-weeks dosage regimen provides further convenience by offering the possibility of synchronising its administration with most chemotherapy regimens. Direct comparisons between approved dosages of darbepoetin alfa and other erythropoietic agents have not been conducted. Such comparisons would be very helpful in formulating definitive conclusions about their relative efficacy and cost effectiveness. Darbepoetin alfa provides an effective and well tolerated treatment option for the treatment of anaemia in patients with cancer receiving chemotherapy.
Pharmacological Properties
Darbepoetin alfa stimulates erythropoiesis by binding to surface receptors on red blood cell (RBC) precursors in the bone marrow and supporting their survival, differentiation and proliferation. Its high sialic acid content results in reduced affinity for the erythropoietin receptor, but a prolonged half-life with resultant enhanced in vivo biological activity compared with rHuEPO in murine models. Subcutaneous darbepoetin alfa was effective in stimulating erythropoiesis in vivo in a murine model of chemoradiotherapy-induced anaemia.
Darbepoetin alfa is slowly absorbed after subcutaneous administration; it has a dose- and time-independent, predictable pharmacokinetic profile. Multiple-dose administration does not result in accumulation. Synchronous versus asynchronous darbepoetin alfa administration with chemotherapy results in increased maximum serum darbepoetin alfa concentration and area under the serum concentration-time curve values. In anaemic patients with cancer, the half-life of subcutaneous darbepoetin alfa (up to 88 hours) is generally longer than that reported for subcutaneous epoetin alfa (40 hours).
Therapeutic Efficacy
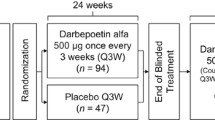
Subcutaneous darbepoetin alfa has shown efficacy in the treatment of anaemia in patients with nonmyeloid malignancies receiving chemotherapy in several randomised trials of double-blind or nonblind design and in a noncomparative trial. The approved regimens of darbepoetin alfa involve starting dosages of 2.25 μg/kg once weekly or 500μg once every 3 weeks, with a provision for a 40% dose reduction if haemoglobin levels increase rapidly or exceed 11 g/dL.
In two well designed trials, darbepoetin alfa 2.25 μg/kg once weekly significantly decreased the incidence of transfusion (weeks 5–13) while producing significantly greater haemoglobin and/or haematopoietic response rates compared with placebo in patients with lung cancer or lymphoproliferative malignancies. In addition, HR-QOL, as demonstrated by improvement in fatigue score associated with anaemia, was significantly improved in darbepoetin alfa recipients.
Darbepoetin alfa 6.75 μg/kg once every 3 weeks for 12 weeks produced an approximately 3-fold higher haemoglobin response rate than placebo in a dose-finding study. The efficacy of darbepoetin alfa 6.75 μg/kg once every 3 weeks was similar when administered synchronously or asynchronously with a chemotherapy regimen given once every 3 weeks.
Darbepoetin alfa 500μg once every 3 weeks was no less effective than darbepoetin alfa 2.25 μg/kg once weekly in terms of the proportion of patients requiring RBC transfusions after 15 weeks’ treatment in patients with nonmyeloid malignancies. HR-QOL results were also similar for the two treatment groups.
Compared with placebo, darbepoetin alfa was also effective at a dosage of 300μg once every 3 weeks in decreasing the incidence of RBC transfusions in cancer patients with chemotherapy-induced anaemia in a well designed trial. These data were supported by the results of a large, single-arm trial (n = 1464) in which the majority of patients receiving darbepoetin alfa 300μg every 3 weeks for 13 weeks achieved a haemoglobin level of ≥11 g/dL and maintained it at 11–13 g/ dL. An early intervention (before the haemoglobin dropped below 10 g/dL) with this regimen was significantly more effective than a late intervention in achieving and maintaining a haemoglobin target level of 11–12 g/dL in a randomised trial in patients with chemotherapy-induced anaemia.
Tolerability
Darbepoetin alfa once weekly or once every 3 weeks was generally well tolerated in patients with solid tumours or lymphoproliferative malignancies when administered subcutaneously at various different dosages in clinical trials of up to 6 months duration. Adverse events reported in clinical trials were generally in accordance with the underlying malignancy and chemotherapy.
Fatigue, diarrhoea and oedema were the most frequent adverse events occurring in controlled clinical trials of darbepoetin alfa. Less frequent, but clinically significant, adverse events included hypertension, seizures/convulsions and thrombotic events, with death (unrelated to treatment) being the most common serious adverse event.
Immunogenic reactions to darbepoetin alfa may occur, and although none of the clinical trials reported neutralising antibodies to darbepoetin alfa, there have been rare cases of pure red cell aplasia in patients treated with darbepoetin alfa in association with neutralising antibodies to erythropoietin.




Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Engert A. Recombinant human erythropoietin in oncology: current status and further developments. Ann Oncol 2005; 16(10): 1584–95
Miller CB, Jones RJ, Piantadosi S, et al. Decreased erythropoietin response in patients with the anemia of cancer. New Eng J Med 1990; 322(24): 1689–92
Cvetkovic RS, Goa KL. Darbepoetin alfa: in patients with chemotherapy-related anaemia. Drugs 2003; 63(11): 1067–74; discussion 1075-7
Ibbotson T, Goa K. Darbepoetin alfa. Drugs 2001 Oct 19; 61(14): 2097–104
Robinson DM, Easthope SE. Darbepoetin alfa: its use in anemia associated with chronic kidney disease. Biodrugs 2005; 19(5): 327–43
Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP). Br J Cancer 2001 Apr, 84 Suppl. 1: 3–10
Egrie JC, Dwyer E, Browne JK, et al. Darbepoetin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietin. Exp Hematol 2003; 31(4): 290–9
Amgen Europe B.V. Product information (EU): Aranesp (darbe-poetin alfa) single-dose vial and single-dose prefilled syringe (8 June 2001) [online]. Available from URL: http://www.emea.eu.int/ [Accessed 2005 Oct 6]
Zamboni WC, Stewart CF. An overview of the pharmacokinetic disposition of darbepoetin alfa. Pharmacotherapy 2002; 22 (9 Pt 2): 133–140S
Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med (Maywood) 2003; 228(1): 1–14
Hartley C, Elliott S, Begley CG, et al. Kinetics of haematopoietic recovery after dose-intensive chemo/radiotherapy in mice: optimized erythroid support with darbepoetin alpha. Br J Haematol 2003; 122(4): 623–36
Amgen Inc. Product information (US): Aranesp (darbepoetin alfa) single-dose vial and single-dose prefilled syringe (24 March 2006) [online]. Available from URL: http://www.aranesp.com [Accessed 2006 Apr 7]
Macdougall IC, Gray SJ, Elston O, et al. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J Am Soc Nephrol 1999 Nov; 10(11): 2392–5
Heatherington AC, Schuller J, Mercer AJ. Pharmacokinetics of novel erythropoiesis stimulating protein (NESP) in cancer patients: preliminary report. Br J Cancer 2001 Apr; 84 Suppl. 1: 11–6
Glaspy J, Henry D, Patel R, et al. Effects of chemotherapy on endogenous erythropoietin levels and the pharmacokinetics and erythropoietic response of darbepoetin alfa: a randomised clinical trial of synchronous versus asynchronous dosing of darbepoetin alfa. Eur J Cancer 2005; 41: 1140–9
Heatherington A, Rovetti R, Kotasek D, et al. Predictability of pharmacokinetic properties of NESP administered once every 3 weeks in patients with nonmyeloid malignancies receiving cyclic chemotherapy [abstract no. 471]. The 37th Annual Meeting of the American Society of Clinical Oncology; May 12–15 2001; San Francisco (CA) 20 Part 1: 119a
Heatherington AC, Schuller J, Mercer AJ, et al. The pharmacokinetics of darbepoetin alfa administered subcutane-ously in patients with non-myeloid malignancies receiving multicycle chemotherapy [abstract no. 1258]. Blood 2001; 98 Suppl.: 298a
Kotasek D, Steger G, Faught W, et al. Darbepoetin alfa administered every 3 weeks alleviates anaemia in patients with solid tumours receiving chemotherapy; results of a double-blind, placebo-controlled, randomised study. Eur J Cancer 2003 Sep; 39(14): 2026–34
Heatherington AC, Dittrich C, Sullivan JT. Pharmacokinetics of darbepoetin alfa after intravenous or subcutaneous administration in patients with non-myeloid malignancies undergoing chemotherapy. Clin Pharmacokinet 2006; 45(2): 199–211
Jelkmann W. The enigma of the metabolic fate of circulating erythropoietin (Epo) in view of the pharmacokinetics of the recombinant drugs rhEpo and NESP. Eur J Haematol 2002; 69(5–6): 265–74
Ortho Biotech Products L.P. Product information (US): Procrit (epoetin alfa) single- and multi-dose vials (January 2005) [online]. Available from URL: http://www.procrit.com [Accessed 2005 Oct 18]
Allon M, Kleinman K, Walczyk M, et al. Pharmacokinetics and pharmacodynamics of darbepoetin alfa and epoetin in patients undergoing dialysis. Clin Pharmacol Ther 2002; 72(5): 546–55
Hedenus M, Adriansson M, San Miguel J, et al. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol 2003 Aug; 122(3): 394–403
Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst 2002 Aug 21; 94(16): 1211–20
Canon JL, Vansteenkiste J, Bodoky G, et al. Randomized, double-blind, active-controlled trial of every-3-week darbepoetin alfa for the treatment of chemotherapy-induced anemia. J Natl Cancer Inst 2006; 98(4): 273–84
Taylor K, Ganly P, Charu V, et al. Randomised, double-blind, placebo-controlled study of darbepoetin alfa every 3 weeks for the treatment of chemotherapy-induced anemia [abstract no. 3556]. The 47th Annual Meeting and Exposition of the American Society of Hematology; 2005 December 10–13; Atlanta (GA)
Rearden TP, Charu V, Saidman B, et al. Results of a randomized study of every three-week dosing (Q3W) of darbepoetin alfa for chemotherapy-induced anemia (CIA). J Clin Oncol 2004; 22 (14 Suppl.): 741. Plus oral presentation made at the 40th Annual Meeting of the American Society of Clinical Oncology; 2004 June 5–8; New Orleans (LA)
Boccia R, Malik IA, Raja V, et al. Darbepoetin alfa administered every three weeks is effective for the treatment of chemotherapy-induced anemia. Oncologist 2006 Apr; 11(4): 409–17
Boccia R, Silberstein P, Tchekmedyian S, et al. The effectiveness of darbepoetin alfa administered every 3 weeks on clinical outcomes in elderly patients with chemotherapy-induced anemia. The First Annual Chicago Supportive Oncology Conference; 2005 October 6–8; Chicago (IL)
Canon JL, Bodoky G, Mateos M, et al. Darbepoetin alfa administered once every three weeks is effective for treating chemotherapy-induced anemia [abstract no. LBA8284] [abstract no. 5033]. The 28th Annual San Antonio Breast Cancer Symposium; 2005 December 8–11; San Antonio (TX)
Hedenus M, Hansen S, Taylor K, et al. Randomized, dose-finding study of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies. Br J Haematol 2002 Oct; 119(1): 79–86
Glaspy JA, Jadeja JS, Justice G, et al. Darbepoetin alfa given every 1 or 2 weeks alleviates anaemia associated with cancer chemotherapy. Br J Cancer 2002 Jul 29; 87(3): 268–76
Canon JL, Vansteenkiste J, Bodoky G, et al. Effect of dose reductions on response to 500mg darbepoetin alfa administered once every 3 weeks for the treatment of chemotherapy-induced anemia: analysis from a randomized, double-blind, active-controlled trial [abstract no. 3558]. The 47th Annual Meeting and Exposition of the American Society of Hematology; 2005 December 10–13; Atlanta (GA)
Canon JL, Vansteenkiste J, Bodoky G, et al. Final results of a randomized, double-blind, active-controlled trial of darbepoetin alfa administered once every 3 weeks for the treatment of anemia in patients receiving multicycle chemotherapy. J Clin Oncol 2005 Jun; 23 (16 Suppl.): 1098
Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst 1999 Oct 6; 91(19): 1616–34
Birgegard G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology 2005; 68 Suppl. 1: 3–11
Engert A. Recombinant human erythropoietin as an alternative to blood transfusion in cancer-related anaemia. Dis Manage Health Outcomes 2000; 8(5): 259–72
Vogelzang NJ, Breitbart W, Cella D, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Semin Hematol 1997; 34 (3 Suppl. 2): 4–12
Cella D, Dobrez D, Glaspy J. Control of cancer-related anemia with erythropoietic agents: a review of evidence for improved quality of life and clinical outcomes. Ann Oncol 2003 Apr; 14(4): 511–9
National Comprehensive Cancer Network (NCCN) cancer and treatment-related anemia panel. NCCN clinical practice guidelines in oncology: cancer and treatment-related anemia: version 1.2006 [online]. Available from URL: http://www.nccn.org [Accessed 2006 Mar 7]
Auerbach M, Ballard H, Trout JR, et al. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol 2004; 22(7): 1301–7
Rizzo JD, Lichtin AE, Woolf SH, et al. Use of epoetin in patients with cancer: evidence-based clinical practice guidelines of the American Society of Clinical Oncology and the American Society of Hematology. Blood 2002; 100(7): 2303–20
Bokemeyer C, Aapro MS, Courdi A, et al. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer. Eur J Cancer 2004; 40(15): 2201–16
Glaspy J, Berg R, Tomita D, et al. Final results of a phase 3, randomized, open-label study of darbepoetin alfa 200 mcg every 2 weeks versus epoetin alfa 40,000 U weekly in patients with chemotherapy-induced anemia [abstract no. 8125]. J Clin Oncol 2005 Jun; 23 (Suppl. 16): 760
Waltzman RJ, Croot C, Justice GR, et al. Randomized comparison of epoetin alfa (40,000U weekly) and darbepoetin alfa (200 mg every 2 weeks) in anemia patients with cancer receiving chemotherapy. Oncologist 2005; 10(8): 642–50
Scidenfeld J, Piper M, Flamm C, et al. Epoetin treatment of anemia associated with cancer therapy: a systematic review and meta-analysis of controlled clinical trials. J Natl Cancer Inst 2001; 93(16): 1204–14
Bohlius J, Langensiepen S, Schwarzer G, et al. Recombinant human erythropoietin and overall survival in cancer patients: results of a comprehensive meta-analysis. J Natl Cancer Inst 2005; 97(7): 489–98
Bohlius J, Wilson J, Bayliss S, et al. Epoetin and darbepoetin to treat cancer patients: updated meta-analysis results [abstract no. 751]. Blood 2005; 106(11): 222a
Hedenus M, Canon JL, Kotasek D, et al. Effects of dose adjustment rules on safety during erythropoietic therapy: a retrospective analysis of darbepoetin alfa administered either every 3 weeks or weekly [abstract no. 3376]. Blood 2005; 106(11): 943a
Caro JJ, Salas M, Ward A, et al. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer 2001; 91(12): 2214–21
Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol 2005; 23(25): 5960–72
Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet 2003; 362(9392): 1255–60
Yasuda Y, Fujita Y, Matsuo T, et al. Erythropoietin regulates tumour growth of human malignancies. Carcinogenesis 2003; 24(6): 1021–9
Elliott S, Busse L, Bass MB, et al. Anti-Epo receptor antibodies do not predict Epo receptor expression. Blood 2006; 107(5): 1892–5
Hedenus M, Vansteenkiste J, Kotasek D, et al. Darbepoetin alfa for the treatment of chemotherapy-induced anemia: disease progression and survival analysis from four randomized, double-blind, placebo-controlled trials. J Clin Oncol 2005 Oct 1; 23(28): 6941–8
Anderson ER, Gibson G. Considerations in darbepoetin alfa cost and reimbursement: a model for pharmacy managers. Pharmacotherapy 2003; 23 (12 Pt 2): 119–24S
Borget I, Tilleul P, Baud M, et al. Routine once-weekly darbepoetin alfa administration is cost-effective in lung cancer patients with chemotherapy-induced anemia: a Markov analysis. Lung Cancer 2006 Mar; 51(3): 369–76
Cremieux P, Greenberg P, Piech CT. Epoetin alfa is more effective and less costly relative to darbepoetin alfa in lung cancer patients receiving treatment for chemotherapy-induced anemia [abstract no. 2248]. The 39th Annual Meeting of the American Society of Clinical Oncology; 2003 May 31–June 3; Chicago (IL) 22: 559
Frame D, Fastenau J, Piech CT. Cost-effectiveness analysis favors epoetin alfa to front-loaded doses of darbepoetin alfa for treatment of chemotherapy-related anemia [abstract]. J Manage Care Pharm 2004 Mar-Apr; 10(2): 180
Rosberg J, Koeller J, Oster E, et al. Clinical response, cost of treatment, and cost-effectiveness of three regimens of two erythropoietic agents for treating chemotherapy-induced anemia [abstract no. 2250]. he 39th Annual Meeting of the American Society of Clinical Oncology; 2003 May 31–June 3; Chicago (IL): 559
Rosberg JH, Ben-Hamadi R, Cremieux PY, et al. Dose conversion and cost effectiveness of erythropoietic therapies in chemotherapy-related anaemia: a meta-analysis. Clin Drug Invest 2005; 25(1): 33–48
Ben-Hamadi R, Duh MS, Aggarwal J, et al. The cost-effectiveness of weekly epoetin alfa relative to weekly darbepoetin alfa in patients with chemotherapy-induced anemia. Curr Med Res Opin 2005 Oct; 21(10): 1677–82
Scarpace SL, Miller K, Elefante A, et al. Cost-utility of darbepoetin alfa (DARBE) on an every-2 week (QOW) schedule in anemic non-myeloid hemataologic malignancies: a positive overall impact on the healthcare system (HCS) [abstract no. 8278]. J Clin Oncol 2004; 22 (14 Suppl.): 797s
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: R. Booton, CRC Department of Medical Oncology, Christie Hospital NHS Trust, Manchester, England; M. Hedenus, Department of Internal Medicine, Sundsvall Hospital, Sundsvall, Sweden; D.H. Henry, Joan Karnell Cancer Center, Pennsylvania Hospital, Philadelphia, Pennsylvania, USA; W.P. McGuire, Weinberg Cancer Institute, Franklin Square Hospital Center, Baltimore, Maryland, USA; R. Pirker, Department of Internal Medicine I, Medical University of Vienna, Vienna, Austria; L.S. Schwartzberg, Hematology and Medical Oncology, The West Clinic, Memphis, Tennessee, USA; J.F. Vansteenkiste, Respiratory Oncology Unit (Pulmonology), Leuven Lung Cancer Group, University Hospital Gasthuisberg, Leuven, Belgium.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘darbepoetin alfa’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE and EMBASE search terms were ‘darbepoetin alfa’ and (‘anaemia’ or ‘anemia’) and (‘cancer’ or ‘oncology’ or ‘chemotherapy’). AdisBase search terms were ‘darbepoetin alfa’ and ‘anaemia’ or ‘anemia’) and (‘cancer’ or ‘oncology’ or ‘chemotherapy’). Searches were last updated 15 May 2006.
Selection: Studies in anaemic patients with cancer who received darbepoetin alfa. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Darbepoetin alfa, anaemia, cancer, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Siddiqui, M.A.A., Keating, G.M. Darbepoetin Alfa. Drugs 66, 997–1012 (2006). https://doi.org/10.2165/00003495-200666070-00018
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200666070-00018