Summary
Abstract
Subcutaneous recombinant interferon-β-1a (Rebif®) 22 or 44μg three times weekly is a valuable option in the first-line treatment in patients with relapsing-remitting multiple sclerosis (RRMS). It has shown benefits on outcome measures related to relapses, progression of disability and magnetic resonance imaging (MRI) in clinical trials. A significant efficacy advantage for subcutaneous interferon-β-1a three times weekly over intramuscular interferon-β-1a 30μg once weekly was shown at 24 and 48 weeks. The most common adverse events are generally mild and clinically manageable. Considering both direct and indirect comparative clinical trial data, an assessment suggests that subcutaneous interferon-β-1a 44μg three times weekly has the best benefit-to-risk values of the available disease-modifying drugs used to treat RRMS.
Pharmacological Properties
Glycosylated recombinant interferon-β-1a has the same primary structure and biological effects in the body as endogenous human interferon-β. Immunomodulatory activity, the principal pharmacodynamic property of interferon-β-1a, most likely results from interferon-stimulated gene products mediating biological activities. The long-term clinical significance of the development of neutralising antibodies (NABs) to interferon-β-1a is currently unclear; therefore, it is recommended that treatment decisions are based on clinical efficacy and not on the presence of NABs alone.
In healthy volunteers, subcutaneous interferon-β-1a has a long apparent elimination half-life and appears to accumulate after repeated doses.
Therapeutic Efficacy
In a randomised 2-year trial, subcutaneous interferon-β-1a 22 or 44μg three times weekly for 2 years was more effective than placebo as assessed by a number of clinical (e.g. relapse rate per patient, proportion of patients without clinical relapse and changes from baseline in Kurtzke Expanded Disability Status Scale [EDSS] scores) and MRI (total burden of disease and numbers of active lesions per patient per scan) endpoints. Significant dose-response effects in favour of the higher dosage were evident when the study period was extended for another 2 years. Extension data for a total follow-up period of 7–8 years in the original cohort showed sustained clinical benefits with subcutaneous interferon-β-1a therapy.
In a 48-week comparative trial, patients randomised to subcutaneous interferon-β-1a 44μg three times weekly were more likely to remain free of relapses and have significantly more favourable measures of MRI lesion activity at weeks 24 and 48 weeks, and a longer time to first relapse, than those randomised to intramuscular interferon-β-1a 30μg once weekly. In the crossover extension phase (median treatment duration 34 weeks), clinical and MRI outcomes improved in patients who crossed over from treatment with intramuscular interferon-β-1a 30μg once weekly to subcutaneous interferon-β-1a 44μg three times weekly.
Tolerability
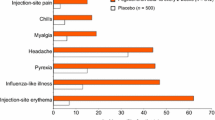
In clinical trials of subcutaneous interferon-β-1a, the most commonly reported adverse events (e.g. injection-site reactions, headache and influenza-like symptoms) were generally mild, manageable and reversible. Mild, asymptomatic and reversible haematological disorders and alterations in liver function tests may occur. A few patients experience serious effects on liver function.
Subcutaneous interferon-β-1a 44μg three times weekly was associated with a significantly higher incidence of injection-site disorders and liver function or haematological abnormalities than intramuscular interferon-β-1a 30μg once weekly. No between-group differences were noted regarding the proportions of patients experiencing serious adverse events.
Pharmacoeconomic and Other Considerations
In an assessment that evaluated the four available RRMS disease-modifying treatments based on the numbers of patients needed to treat to obtain benefit or harm, subcutaneous interferon-β-1a 44μg three times weekly had the best benefit-to-risk values. Based on data from pivotal directly comparative trials, subcutaneous interferon-β-1a 44μg three times weekly had favourable benefit-to-risk values relative to subcutaneous interferon-β-1a 22μg three times weekly and intramuscular interferon-β-1a 30μg once weekly. Based on indirect comparative data from published clinical trials, subcutaneous interferon-β-1a appeared to have favourable efficacy values relative to interferon-β-1b every other day, and interferon-β formulations appeared to have favourable benefit-to-risk ratios relative to placebo and also subcutaneous glatiramer acetate 20gmg once daily.
In pharmacoeconomic analyses from a healthcare payer perspective, subcutaneous interferon-β-1a 44μg three times weekly was predicted to be increasingly more cost effective than placebo over the mid-(10 years) and long-(20 years) term with regard to preventing EDSS-months of disability in the UK and France, and cost saving relative to intramuscular interferon-β-1a 30μg once weekly over 48 weeks in the US.








Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Khan O, Zabad R, Caon C, et al. Comparative assessment of immunomodulating therapies for relapsing-remitting multiple sclerosis. CNS Drugs 2002; 16(8): 563–78
O’Connor P. Key issues in the diagnosis and treatment of multiple sclerosis: an overview. Neurology 2002 Sep 24; 59 (6 Suppl. 3): S1–33
Goodin DS, Frohman EM, Garmany Jr GP, et al. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology 2002 Jan 22; 58(2): 169–78
Corboy JR, Goodin DS, Frohman EM. Disease-modifying therapies for multiple sclerosis. Curr Treat Options Neurol 2003 Jan; 5(1): 35–54
Beta interferon and glatiramer acetate for the treatment of multiple sclerosis. Technology Appraisal Guidance No. 32. London: National Institute for Clinical Excellence, 2002 Jan
Polman CH, Uitdehaag BMJ. Drug treatment of multiple sclerosis. BMJ 2000 Aug 19; 321: 490–4
Fernandez O. Interferons in relapsing-remitting multiple sclerosis: are there benefits from long-term use? CNS Drugs 2004; 18(15): 1057–70
Freedman MS, Blumhardt LD, Brochet B, et al. International consensus statement on the use of disease-modifying agents in multiple sclerosis. Mult Scler 2002 Feb; 8(1): 19–23
Biogen Idec Inc., Elan Pharmaceuticals. Important drug warning: voluntary suspension of Tysabri (natalizumab) marketing [online]. Available from URL: http://www.tysabri.com [Accessed 2005 Mar 22]
Serono, Inc. Rebif (interferon-beta-1a) sc injection: prescribing information. Rockland (MA): Serono, Inc., 2004
Liberati AM, Horisberger MA, Palmisano L, et al. Double-blind randomized phase I study on the clinical tolerance and biological effects of natural and recombinant interferon-β. J Interferon Res 1992; 12(5): 329–36
Liberati AM, Garofani P, De Angelis V, et al. Double-blind randomized phase I study on the clinical tolerance and pharmacodynamics of natural and recombinant interferon-β given intravenously. J Interferon Res 1994; 14(2): 61–9
Dhib-Jalbut S. Mechanisms of action of interferons and glatiramer acetate in multiple sclerosis. Neurology 2002 Apr 23; 58 (8 Suppl. 4): S3–9
Zhang J, Hutton G, Zang Y. A comparison of the mechanisms of action of interferon beta and glatiramer acetate in the treatment of multiple sclerosis. Clin Ther 2002 Dec; 24(12): 1998–2021
Wagstaff AJ, Goa KL. Recombinant interferon-β-1a: a review of its therapeutic efficacy in relapsing-remitting multiple sclerosis. Biodrugs 1998; 10(6): 471–94
Rothuizen LE, Buclin T, Spertini F, et al. Influence of interferon β-1a dose frequency on PBMC cytokine secretion and biological effect markers. J Neuroimmunol 1999 Sep 1; 99(1): 131–41
Buraglio M, Trinchard-Lugan I, Munafo A, et al. Recombinant human interferon-β-1a (Rebif) vs recombinant interferon-β-1b (Betaseron) in healthy volunteers: a pharmacodynamic and tolerability study. Clin Drug Invest 1999; 18(1): 27–34
Salmon P, Le Cotonnec JY, Galazka A, et al. Pharmacokinetics and pharmacodynamics of recombinant human interferon-β in healthy male volunteers. J Interferon Cytokine Res 1996 Oct; 16(10): 759–64
Munafo A, Trinchard-Lugan I, Nguyen TX, et al. Comparative pharmacokinetics and pharmacodynamics of recombinant human interferon beta-1a after intramuscular and subcutaneous administration. Eur J Neurol 1998 Mar; 5(2): 187–93
Buchwalder PA, Buclin T, Trinchard I, et al. Pharmacokinetics and pharmacodynamics of IFN-β1a in healthy volunteers. J Interferon Cytokine Res 2000 Oct; 20(10): 857–66
Bertolotto A, Gilli F, Sala A, et al. Evaluation of bioavailability of three types of IFNβ in multiple sclerosis patients by a new quantitative-competitive-PCR method for MxA quantification. J Immunol Methods 2001 Oct 1; 256(1–2): 141–52
Deisenhammer F, Mayringer I, Harvey J, et al. A comparative study of the relative bioavailability of different interferon beta preparations. Neurology 2000 Jun 13; 54(11): 2055–60
Galboiz Y, Shapiro S, Lahat N, et al. Matrix metalloproteinases and their tissue inhibitors as markers of disease subtype and response to interferon-β therapy in relapsing and secondary-progressive multiple sclerosis patients. Ann Neurol 2001 Oct; 50(4): 443–51
Brescia Morra V, Coppola G, Orefice G, et al. Interferon-β treatment decreased cholesterol plasma levels in multiple sclerosis patients. Neurology 2004 Mar; 62 Pt 1: 829–30
Wandinger KP, Lunemann JD, Wengert O, et al. TNF-related apoptosis inducing ligand (TRAIL) as a potential response marker for interferon-beta treatment in multiple sclerosis. Lancet 2003 Jun 14; 361: 2036–43
Sharief MK, Semra YK. Down-regulation of survivin expression in T lymphocytes after interferon beta-1a treatment in patients with multiple sclerosis. Arch Neurol 2002 Jul; 59(7): 1115–21
Harzheim M, Stepien-Mering M, Schroder R, et al. The expression of microfilament-associated cell-cell contacts in brain endothelial cells is modified by IFN-β1a (Rebif). J Interferon Cytokine Res 2004 Dec; 24(12): 711–6
Hong J, Tejada-Simon MV, Rivera VM, et al. Anti-viral properties of interferon beta treatment in patients with multiple sclerosis. Mult Scler 2002 May; 8(3): 237–42
Rice G. The significance of neutralizing antibodies in patients with multiple sclerosis treated with interferon beta. Arch Neurol 2001 Aug; 58(8): 1297–8
Bertolotto A, Gilli F, Sala A, et al. Persistent neutralizing antibodies abolish the interferon β bioavailability in MS patients. Neurology 2003 Feb 25; 60(4): 634–9
Ross C, Clemmesen KM, Svenson M, et al. Immunogenicity of interferon-β in multiple sclerosis patients: influence of preparation, dosage, dose frequency, and route of administration. Danish Multiple Sclerosis Study Group. Ann Neurol 2000 Nov; 48(5): 706–12
Bertolotto A, Deisenhammer F, Gallo P, et al. Immunogenicity of interferon beta: differences among products. J Neurol 2004 Jun; 251 Suppl. 2: ll/15–24
Perini P, Facchinetti A, Bulian P, et al. Interferon-beta (INF-β) antibodies in interferon-β 1a-and interferon-β1b-treated multiple sclerosis patients: prevalence, kinetics, cross-reactivity, and factors enhancing interferon-β immunogenicity in vivo. Eur Cytokine Netw 2001 Mar; 12(1): 56–61
Vartanian TK, Zamvil SS, Fox E, et al. Neutralizing antibodies to disease-modifying agents in the treatment of multiple sclerosis. Neurology 2004 Dec 14; 63 (11 Suppl. 5): S42–9
Panitch H, Goodin DS, Francis G, et al. Randomized, comparative study of interferon β-1a treatment regimens in MS: the EVIDENCE Trial. Neurology 2002 Nov 26; 59(10): 1496–506
Paty D. Long-term observational efficacy and safety follow-up of the PRISMS cohort [abstract no. P555 plus poster]. 19th Congress of the European Committee for Treatment and Research in Multiple Sclerosis; 2003 Sep 17–20; Milan
Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis: PRISMS (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 1998; 352 (9139): 1498–504
Schwid SR, Thorpe J, Sharief M, et al. Enhanced benefit of increasing interferon beta-1a dose and frequency in relapsing MS: the EVIDENCE study. Arch Neurol 2005 May; 62(5): 785–92
PRISMS-4: long-term efficacy of interferon-β-1a in relapsing MS. Neurology 2001 Jun 26; 56 (12): 1628–36
Sorensen PS, Ross C, Clemmesen KJ, et al. Clinical importance of neutralising antibodies against interferon beta in patients with relapsing-remitting multiple sclerosis. Lancet 2003 Oct 11; 362(9391): 1184–91
Li D, Abdalla JA. Long-term observational follow-up of the PRISMS cohort: analyses of MRI BOD shows benefit of high dose, high frequency IFNbeta-1a (Rebif) [abstract no. P02.118]. Neurology 2004 Apr; 62 (7 Suppl. 5): A153–4
Li DK, Paty DW. Magnetic resonance imaging results of the PRISMS trial: a randomized, double-blind, placebo-controlled study of interferon-β1a in relapsing-remitting multiple sclerosis. Ann Neurol 1999 Aug; 46(2): 197–206
Francis GS, Grumser Y, Alteri E, et al. Heptatic reactions during treatment of multiple sclerosis with inteferon-β-1a: incidence and clinical significance. Drug Saf 2003; 26(11): 815–27
Tremlett HL, Oger J. Elevated aminotransferases during treatment with interferon-beta for multiple sclerosis: actions and outcomes. Mult Scler 2004 Jun; 10(3): 298–301
Tremlett HL, Yoshida EM, Oger J. Liver injury associated with the β-interferons for MS: a comparison between the three products. Neurology 2004 Feb 24; 62(4): 628–31
Tremlett H, Oger J. Hepatic injury, liver monitoring and the beta-interferons for multiple sclerosis. J Neurol 2004 Nov; 251(11): 1297–303
Rieckmann P, O’Connor P, Francis GS, et al. Haematological effects of interferon-β-1a (Rebif) therapy in multiple sclerosis. Drug Saf 2004; 27(10): 745–56
Patten SB, Metz LM. Interferon β-1a and depression in relapsing-remitting multiple sclerosis: an analysis of depression data from the PRISMS clinical trial. Mult Scler 2001 Aug; 7(4): 243–8
Sandberg-Wollheim M, Bever C, Carter J, et al. Comparative tolerance of IFN beta-1a regimens in patients with relapsing multiple sclerosis: the EVIDENCE study. J Neurol 2005 Jan; 252(1): 8–13
Francis GS. Importance of benefit-to-risk assessment for disease-modifying drugs used to treat MS. J Neurol 2004 Sep; 251 Suppl. 5: v/42–9
Lepen C, Coyle P, Vollmer T, et al. Long-term cost effectiveness of interferon-β-1a in the treatment of relapsing-remitting multiple sclerosis: an econometric model. Clin Drug Invest 2003; 23(9): 571–81
Beresniak A., Coyle P, Vollmer T, et al. Cost-benefit and cost-effectiveness analyses of interferon beta-1a therapies for multiple sclerosis [abstract no. P414 plus poster]. 13th Annual Meeting of the European Neurological Society; 2003 Jun 14–18; Istanbul
Galetta SL, Markowitz C, Lee AG. Immunomodulatory agents for the treatment of relapsing multiple sclerosis: a systematic review. Arch Intern Med 2002 Oct 28; 162(19): 2161–9
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: C. Gasperini, Department of Neuroscience, S. Camillo-Forlanini Hospital, Rome, Italy; R.M. Herndon, Department of Neurology, G.V. (Sonny) Montgomery Department of Veterans Affairs Medical Center, Jackson, Mississippi, USA; D.D. Miksikostas, Department of Neurology, Athens Naval Hospital, Athens, Greece; H.S. Panitch, Neurology Health Care Service, University of Vermont, College of Medicine, Burlington, Vermont, USA; P.S. Sorensen, Department of Neurology, Copenhagen University Hospital, Copenhagen, Denmark.
Data Selection
Sources: Medical literature published in any language since 1980 on interferon-beta-1a, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE search terms were ‘interferon-beta-1a’. EMBASE search terms were ‘interferon-beta-1a’ or ‘IFN-beta-1a’. AdisBase search terms were ‘interferon-beta-1a’ or ‘IFN-beta-1a’. Searches were last updated 11 May 2005.
Selection: Studies in patients with multiple sclerosis who received interferon-β-1a. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Interferon-β-1a, multiple sclerosis, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Murdoch, D., Lyseng-Williamson, K.A. Subcutaneous Recombinant Interferon-β-1a (Rebif®). Drugs 65, 1295–1312 (2005). https://doi.org/10.2165/00003495-200565090-00010
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200565090-00010