Summary
Abstract
Eprosartan is a potent and selective angiotensin II subtype 1 receptor antagonist. Results of large (n > 100) randomised double-blind studies in patients with mild, moderate or severe hypertension demonstrated that the antihypertensive efficacy of eprosartan (usually 400 to 800 mg/day as a single daily dose or in 2 divided doses) is significantly greater than that of placebo and at least as good as that of enalapril. In placebo-controlled trials, eprosartan achieved mean reductions from baseline in trough sitting systolic blood pressure of 6.3 to 15mm Hg and in diastolic blood pressure of 4.1 to 9.7mm Hg. Response rates associated with once daily administration of eprosartan 400 to 800mg were approximately double those with placebo.
Overall, eprosartan was well tolerated with a similar tolerability profile to that of placebo. In comparative trials, in which the incidence of persistent dry cough was evaluated as the primary end-point, enalapril was several-fold more likely to induce this adverse event than eprosartan (the difference being statistically significant regardless of study population and definition of cough).
In conclusion, the angiotensin II receptor antagonist eprosartan is a well tolerated and effective antihypertensive agent that is administered once or twice daily without regard to meals. Eprosartan has a low potential for serious adverse events, and the drug has not been associated with clinically significant drug interactions. Unlike ACE inhibitors such as enalapril, eprosartan does not have a high propensity to cause persistent nonproductive cough. Thus, eprosartan represents a useful therapeutic option in the management of patients with hypertension.
Pharmacodynamic Properties
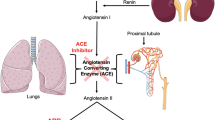
Eprosartan is a potent and selective antagonist of the angiotensin II subtype 1 (AT1) receptor, which mediates the well known effects of angiotensin II, such as vasoconstriction, aldosterone release and renal sodium handling. In animal tissues, high concentrations of eprosartan had no affinity for any other receptor types [e.g. angiotensin II subtype 2 (AT2), adrenergic, serotonergic]. AT2 receptor antagonists such as eprosartan have no effect on the breakdown of peptides such as bradykinin, whereas ACE inhibitors are associated with bradykinin potentiation, an effect that may be implicated in the development of persistent nonproductive cough.
Functional antagonism of AT1 receptor-mediated activity was demonstrated in a variety of in vitro analyses, using animal tissues and cloned human AT1 receptors, and in several in vivo studies in animal models and in healthy volunteers and patients with hypertension. Overall, results of these studies demonstrated that eprosartan inhibited various physiological effects associated with angiotensin II (e.g. contractile responses in rabbit aortic rings, isotonic fluid absorption in rabbit perfused proximal convoluted tubules, pressor responses in animal models and healthy volunteers), and demonstrated true competitive antagonism in studies that investigated this characteristic. Increasing doses of other AT1 receptor antagonists produced a rightward shift of the angiotensin concentration-response curve but, unlike eprosartan, also reduced the maximum responses to angiotensin II. In a rat model of severe hypertension, eprosartan markedly reduced mortality and ECG evidence of cardiac hypertrophy.
Eprosartan had equal affinity for postsynaptic (vascular) and presynaptic (neuronal) AT1 receptors in in vitro studies, whereas losartan inhibited only postsynaptic AT1 receptors. In in vivo studies involving single dose parenteral administration of eprosartan and other AT1 receptor antagonists to small numbers of pithed rats, only eprosartan inhibited presynaptic (as well as postsynaptic) sympathetic outflow. However, whether these findings translate into a potential clinical advantage of eprosartan over other agents has yet to be demonstrated.
In healthy volunteers with an activated renin-angiotensin system or in those receiving exogenous angiotensin II, eprosartan reduced blood pressure and plasma aldosterone levels, and increased urinary sodium excretion, renal plasma flow and plasma renin activity. In patients with hypertension or renal insufficiency, eprosartan had no effect on glomerular filtration rate, renal plasma flow or sodium excretion. Eprosartan (unlike losartan) does not appear to have uricos-uric activity.
Pharmacokinetic Properties
The pharmacokinetic properties of eprosartan after oral administration have been determined primarily in healthy adults, although limited data in patients with hypertension appear to be similar. Oral bioavailability is approximately 13% and is not significantly affected by food. Time to achieve maximum plasma drug concentration ranges from 1 to 3 hours depending on food intake (i.e. food tends to delay eprosartan absorption). Eprosartan has a volume of distribution of approximately 13L and is highly bound to plasma proteins (∼98%). Approximately two-thirds of eprosartan reaching the systemic circulation is eliminated unchanged by biliary excretion, the remaining one-third is eliminated in the urine primarily as unchanged drug. Approximately 20% of renal elimination of eprosartan is as the acyl glucuronide. The drug has an elimination half-life of approximately 4.5 to 9 hours.
No differences were noted in the pharmacokinetic properties of eprosartan between healthy men and women, but area under the concentration-time curve (AUC0–∞) and peak plasma concentration (Cmax) were about twice as high in elderly versus younger volunteers. AUC values were also increased by about 40 to 50% in patients with chronic liver disease and by approximately 25 to 55% in patients with moderate to severe renal impairment compared with healthy volunteers. However, in view of the good tolerability profile of the drug, none of these differences were deemed significant enough to warrant initial dosage adjustments of eprosartan.
Eprosartan is not metabolised by the cytochrome P450 (CYP) enzyme system and in vitro studies indicate that the drug does not inhibit a variety of CYP isoenzymes (e.g. CYP2C9, CYP3A); therefore, the drug has a low potential for pharmacokinetic drug interactions. Indeed, several studies (typically in healthy volunteers but sometimes in patients with specific disorders such as type 2 diabetes mellitus) have been undertaken evaluating the effect of eprosartan on plasma concentrations or pharmacodynamic effects of other drugs [e.g. digoxin, warfarin, glibenclamide (glyburide)]. Eprosartan was found to have no clinically significant interactions. Studies of concomitant administration of known CYP inhibitors (e.g. fluconazole, ketoconazole) also showed no effect of these drugs on the pharmacokinetics of eprosartan.
Therapeutic Efficacy
Results of randomised, double-blind, placebo-controlled, multicentre trials in >100 patients consistently demonstrated statistically significant differences in antihypertensive efficacy favouring eprosartan 400 to 800 mg/day (as a single daily dose or in 2 divided doses) over placebo. Eprosartan achieved mean reductions from baseline in trough sitting diastolic blood pressure (DBP; primary efficacy end-point) ranging from 4.1 to 9.7mm Hg and corresponding reductions in systolic blood pressure (SBP) of 6.3 to 15mm Hg. Mean reductions in sitting DBP with placebo were 0.1 to 4mm Hg and mean changes in sitting SBP ranged from a small increase of 0.9mm Hg to a reduction of 4mm Hg. Response rates (percentage of patients with sitting DBP <90mm Hg or ≥10mm Hg reduction from baseline) were approximately twice as high with once daily administration of eprosartan than placebo (42 and 47% with eprosartan vs 21 or 26% with placebo; p < 0.05 for both comparisons) in 2 studies evaluating this parameter.
In 3 randomised comparisons with enalapril, the main efficacy results showed that eprosartan is at least as effective as enalapril, whether used as monotherapy or in combination with hydrochlorothiazide. In the largest study (n = 528) eprosartan (200 or 300mg twice daily) and enalapril (5 to 20mg once daily) were similarly effective at reducing sitting SBP/DBP after 26 weeks of therapy (15.5/12.9 vs 14.7/11.9mm Hg), but the antihypertensive response rate was significantly greater with eprosartan at both 12 (70 vs 63%; p < 0.05) and 26 (82 vs 73%; p < 0.05) weeks. Approximately 30% of patients in both treatment groups required concomitant hydrochlorothiazide therapy. It is noteworthy, however, that in this and 1 of the other comparative trials, the primary end-point was the incidence of persistent cough. Likewise, antihypertensive efficacy parameters were secondary end-points in a smaller 4-week comparison between eprosartan and losartan (n = 60), in which blood pressure reductions and response rate tended to favour eprosartan (not statistically significant).
Various subgroup analyses of larger studies comparing eprosartan with placebo or enalapril showed good antihypertensive activity with eprosartan regardless of age or gender. Eprosartan also provided a more consistent antihypertensive effect than enalapril in African-American patients on the basis of a small retrospective subgroup analysis.
Tolerability
Adverse events were reported by a similar proportion of patients with hypertension receiving eprosartan or placebo (54.4 vs 52.8%) and the rate of discontinuation of therapy because of adverse events was also similar (4 vs 6.5%) according to pooled data from placebo-controlled clinical trials (n = 1554). The most frequently reported adverse events with both eprosartan and placebo in these studies were headache (10.1 vs 10.8%), upper respiratory tract infection (7.9 vs 5.4%), myalgia (4.0 vs 4.0%), rhinitis (4.0 vs 2.9%), pharyngitis (3.7 vs 2.6%) and cough (3.5 vs 2.6%).
Comparative trials with enalapril showed that both drugs were similarly well tolerated. However, the incidence of persistent, dry, unproductive cough was several-fold higher (varied widely depending on the study population and definition of cough) among enalapril recipients in 2 studies for which this parameter was the primary end-point. The differences between treatment groups was statistically significant for both strict and less rigid definitions of persistent cough. One of the trials showed a 90% relative reduction in ‘definite’ cough when eprosartan was compared with enalapril in 111 patients with a history of enalapril-induced cough (2.6 vs 25.0%; p < 0.01).
Dosage and Administration
The usual recommended starting dosage of eprosartan is 600mg once daily in patients with hypertension receiving the drug as monotherapy. The recommended dosage range is 400 to 800 mg/day administered as a single daily dose or as 2 divided doses. If necessary, the drug may be used in combination with other antihypertensive drugs (e.g. thiazide diuretics or calcium antagonists).
In general, initial dosage adjustments of eprosartan are not necessary in patients with renal or hepatic impairment or in elderly patients. Patients who are volume-and/or salt-depleted (e.g. from diuretics) may develop symptomatic hypotension, and these conditions should be corrected prior to administration of eprosartan.









Similar content being viewed by others
References
McClellan KJ, Balfour JA. Eprosartan. Drugs 1998 May; 55: 713–8
Peach MJ. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev 1977; 57: 313–70
Materson BJ, Preston RA. Angiotensin-converting enzyme inhibitors in hypertension: a dozen years of experience. Arch Intern Med 1994; 154: 513–23
Edwards RM, Ruffolo JRR, Brooks DP. Pharmacology of the angiotensin II receptor antagonist, eprosartan. Expert Opin Invest Drug 1998 Mar; 7: 463–9
Brooks DP, Ohlstein EH, Ruffolo Jr RR. Pharmacology of eprosartan, an angiotensin II receptor antagonist: exploring hypotheses from clinical data. Am Heart J 1999 Sep; 138 (3 Pt 2): S246–51
Goa KL, Wagstaff AJ. Losartan potassium: a review of its pharmacology, clinical efficacy and tolerability in the management of hypertension. Drugs 1996; 51(5): 820–45
Campbell DJ. Angiotensin converting enzyme (ACE) inhibitors and kinin metabolism: evidence that ACE inhibitors may inhibit a kininase other than ACE. Clin Exp Pharmacol Physiol 1995; 22(12): 903–11
Edwards RM, Aiyar N. Angiotensin II receptor subtypes in the kidney. J Am Soc Nephrol 1993; 3: 1643–52
Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. Ann Intern Med 1992; 117: 234–42
Semple PF. Putative mechanisms of cough after treatment with angiotensin converting enzyme inhibitors. J Hypertens 1995; 13 Suppl. 3: S17–21
Edwards RM, Horiuchi M. Recent developments in angiotensin II receptor research. Pharmacol Commun 1996; 7: 341–8
McConnaughey MM, McConnaughey JS, Ingenito AJ. Practical considerations of the pharmacology of angiotensin receptor blockers. J Clin Pharmacol 1999; 39(6): 547–59
Timmermans PB, Smith RD. Angiotensin II receptor subtypes: selective antagonists and functional correlates. Eur Heart J 1994; 15 Suppl. D: 79–87
Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet 2000 Feb 19; 355: 637–45
Timmermans PB, Wong PC, Chiu AT, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev 1993; 45: 205–50
Edwards RM, Aiyar N, Ohlstein EH, et al. Pharmacological characterization of the nonpeptide angiotensin II receptor antagonist, SK & F 108566. J Pharmacol Exp Ther 1992 Jan; 260: 175–81
Edwards RM, Stack EJ, Weidley EF, et al. Characterization of renal angiotensin II receptors using subtype selective antagonists. J Pharmacol Exp Ther 1992 Mar; 260: 933–8
Aiyar N, Griffin E, Shu A, et al. Characterization of [3H]SK & F 108566 as a radioligand for angiotensin type-1 receptor. J Recept Res 1993; 13: 849–61
Edwards RM, Stack EJ. Angiotensin II inhibits glomerular ad-enylate cyclase via the AT1 receptor subtype. J Pharmacol Exp Ther 1993; 266: 506–10
Aiyar N, Baker E, Wu H-L, et al. Human AT1 receptor is a single copy gene: characterization in a stable cell line. Mol Cell Biochem 1994; 131: 75–86
Edwards RM, Trizna W, Stack EJ, et al. Interaction of nonpeptide angiotensin II receptor antagonists with the urate transporter in rat renal brush-border membranes. J Pharmacol Exp Ther 1996 Jan; 276: 125–9
Roch-Ramel F, Guisan B, Diezi J. Effects of uricosuric and antiuricosuric agents on urate transport in human brush-border membrane vesicles. J Pharmacol Exp Ther 1997; 280: 839–45
Hieble JP, McCafferty GM, Sulpizio AC. Effects of losartan and eprosartan on prejunctional angiotensin II receptors in the rabbit ear artery [abstract no. O.50]. J Hum Hypertens 1999 May; 13 Suppl. 3: S38
Brooks DP, Fredrickson TA, Weinstock J, et al. Antihypertensive activity of the non-peptide angiotensin II receptor antagonist, SK & F 108566, in rats and dogs. Naunyn Schmiedebergs Arch Pharmacol 1992 Jun; 345: 673–8
Barone FC, Chandra S, Nelson A, et al. Eprosartan improves ultrasound and MRI indices of cardiac function, prevents renal dysfunction and reduces mortality in severe hypertension [abstract no. P.25]. J Hum Hypertens 1999 May; 13 Suppl. 3: 7
Chandra S, Willette RN, Sauennelch CF, et al. Eprosartan increases vascular distensibility in a rat model of severe hyper-tension [abstract no. P.26]. J Hum Hypertens 1999 May; 13 Suppl. 3: 7
Ohlstein EH, Brooks DP, Feuerstein GZ, et al. Inhibition of sympathetic outflow by the angiotensin II receptor antagonist, eprosartan, but not by losartan, valsartan or irbesartan: relationship to differences in prejunctional angiotensin II receptor blockade. Pharmacology 1997 Nov; 55: 244–51
Brooks DP, DePalma PD. Potential impact of competitive vs. insurmountable angiotensin II receptor antagonism on renal function [abstract no. P.21]. J Hum Hypertens 1999 May; 13 Suppl. 3: S6
Brooks DP, DePalma PD, Ruffolo Jr RR. Effect of captopril and the nonpeptide angiotensin II antagonists, SK & F 108566 and EXP3174, on renal function in dogs with a renal artery stenosis. J Pharmacol Exp Ther 1992 Nov; 263: 422–7
Wang YX, Brooks DP. Renin-angiotensin system inhibition reduces glycine-induced glomerular hyperfiltration in conscious rats. J Pharmacol Exp Ther 1992 Apr; 261: 96–100
Eprosartan (Teveten) prescribing information. Buffalo Grove (IL): Unimed Pharmaceuticals, Inc. A Solvay Pharmaceuticals, Inc. Company. Dec 1999
Price DA, De’Oliveira JM, Fisher NDL, et al. Renal hemodynamic response to an angiotensin II antagonist, eprosartan, in healthy men. Hypertension 1997 Aug; 30 (Pt 1): 240–6
Kazierad DJ, Ilson B, Jorkasky D. Overview of the renal hemodynamic effects of eprosartan, a novel angiotensin II receptor antagonist [abstract no. P.29]. J Hum Hypertens 1999 May; 13 Suppl. 3: 8
Ilson BE, Boike SC, Martin DE, et al. A dose-response study to assess the renal hemodynamic, vascular, and hormonal effects of eprosartan, an angiotensin II AT1-receptor antagonist, in sodium-replete healthy men. Clin Pharmacol Ther 1997 Apr; 63: 471–81
Boike S, Ilson B, Audet P, et al. The angiotensin II receptor antagonist SK & F 108566 does not increase uric acid (UA) excretion in healthy men [abstract no. 45P]. J Am Soc Nephrol 1993; 54(3): 530
Diamond JA, Gharavi A, Roychoudhury D, et al. Effect of long-term eprosartan versus enalapril antihypertensive therapy on left ventricular mass and coronary flow reserve in stage I-II hypertension. Curr Med Res Opin 1999; 15(1): 1–8
Gavras I, Gavras H, Eprosartan Multinational Study Group. Effects of eprosartan versus enalapril in hypertensive patients on the renin-angiotensin-aldosterone system and safety parameters: results from a 26-week, double-blind, multicentre study. Curr Med Res Opin 1999; 15(1): 15–24
Nakashima M, Uematsu T, Kosuge K, et al. Pilot study of the uricosuric effect of DuP-753, a new angiotensin II receptor antagonist, in healthy subjects. Eur J Clin Pharmacol 1992; 42: 333–5
Sica DA, Hollenberg NK. The renal profile of eprosartan. Pharmacotherapy 1999 Apr; 19 (Pt 2): 86S–94S
Tenero D, Martin D, Ilson B, et al. Pharmacokinetics of intravenously and orally administered eprosartan in healthy males: absolute bioavailability and effect of food. Biopharm Drug Dispos 1998; 19: 351–6
Chapelsky MC, Martin DE, Tenero DM, et al. A dose proportionality study of eprosartan in healthy male volunteers. J Clin Pharmacol 1998; 38: 34–9
Kovacs SJ, Tenero DM, Martin DE, et al. Pharmacokinetics and protein binding of eprosartan in hemodialysis-dependent patients with end-stage renal disease. Pharmacotherapy 1999 May; 19: 612–9
Martin DE, Chapelsky MC, Ilson B, et al. Pharmacokinetics and protein binding of eprosartan in healthy volunteers and in patients with varying degrees of renal impairment. J Clin Pharmacol 1998 Feb; 38: 129–37
Tenero D, Martin D, Chapelsky M, et al. Effect of hepatic disease on the pharmacokinetics and plasma protein binding of eprosartan. Pharmacotherapy 1998; 18: 42–50
Tenero DM, Martin DE, Miller AK, et al. Effect of age and gender on the pharmacokinetics of eprosartan. Br J Clin Pharmacol 1998 Sep; 46: 267–70
Bottorff MB, Tenero DM. Pharmacokinetics of eprosartan in healthy subjects, patients with hypertension, and special populations. Pharmacotherapy 1999 Apr; 19 (4 Pt 2): 73S–8S
Cox PJ, Bush BD, Gorycki PD, et al. The metabolic fate of eprosartan in healthy subjects. Exp Toxicol Pathol 1996; 48 Suppl. II: 75–82
Blum RA, Kazierad DJ, Tenero DM. A review of eprosartan pharmacokinetic and pharmacodynamic drug interaction studies. Pharmacotherapy 1999 Apr; 19 (4 Pt 2): 79S–85S
Martin DE, Tompson D, Boike SC, et al. Lack of effect of eprosartan on the single dose pharmacokinetics of orally administered digoxin in healthy male volunteers. Br J Clin Pharmacol 1997 Jun; 43: 661–4
Tenero DM, Martin DE, Ilson BE, et al. Effect of ranitidine on the pharmacokinetics of orally administered eprosartan, an angiotensin II antagonist, in healthy male volunteers. Ann Pharmacother 1998 Mar; 32: 304–8
Kazierad DJ, Martin DE, Blum RA, et al. Effect of fluconazole on the pharmacokinetics of eprosartan and losartan in healthy male volunteers. Clin Pharmacol Ther 1997 Oct; 62: 417–25
Martin DE, DeCherney GS, Ilson BE, et al. Eprosartan, an angiotensin II receptor antagonist, does not affect the pharmacodynamics of glyburide in patients with type II diabetes mellitus. J Clin Pharmacol 1997 Feb; 37: 155–9
Kazierad DJ, Martin DE, Ilson B, et al. Eprosartan does not affect the pharmacodynamics of warfarin. J Clin Pharmacol 1998 Jul; 38: 649–53
Guidelines Subcommittee of the WHO-ISH. 1999 World Health Organization —International Society of Hypertension Guidelines for the Management of Hypertension. J Hypertens 1999 Feb; 17: 151–83
Ramsay LE, Williams B, Johnston GD, et al. British Hypertension Society guidelines for hypertension management 1999: summary. BMJ 1999 Sep 4; 319: 630–5
Ramsay LE, Williams B, Johnston GD, et al. Guidelines for management of hypertension: report of the third working party of the British Hypertension Society. J Hum Hypertens 1999 Sep; 13: 569–92
Sega R. Efficacy and safety of eprosartan in severe hypertension. Blood Press 1999 Mar; 8: 114–21
Elliott WJ, Eprosartan Study Group. Double-blind comparison of eprosartan and enalapril on cough and blood pressure in unselected hypertensive patients. J Hum Hypertens 1999 Jun; 13: 413–7
Gradman AH, Gray J, Maggiacomo F, et al. Assessment of once-daily eprosartan, an angiotensin II antagonist, in patients with systemic hypertension. Clin Ther 1999 Mar; 21: 442–53
Hedner T, Himmelmann A, Eprosartan Multinational Study Group. The efficacy and tolerance of one or two daily doses of eprosartan in essential hypertension. J Hypertens 1999 Jan; 17: 129–36
Weber M. Efficacy and safety of eprosartan in patients with essential hypertension: results of an 8-week, double-blind, placebo-controlled, multicenter trial [abstract no. P31.075]. J Hypertens 1998 Jun; 16 Suppl. 2: 245
White WB, Anis Anwar Y, Sica DA. Once-daily effects of the angiotensin receptor blocker, eprosartan on 24-hour blood pressure in patients with systemic hypertension [abstract]. Am J Hypertens 1999 Apr; 12 (4 Pt 2): 27A
White WB, Mansoor GA, Lessem J, et al. Assessment of the angiotensin II receptor antagonist eprosartan, by office and ambulatory BP monitoring [abstract no. C17]. Am J Hypertens 1995; 8 (4 Pt 2): 179A
Meredith PA. Role of trough to peak efficacy in the evaluation of antihypertensive therapy. J Hypertens 1998 Jan; 16 Suppl. 1: S59–64
Oparil S. Eprosartan versus enalapril in hypertensive patients with angiotensin-converting enzyme inhibitor-induced cough. Curr Ther Res Clin Exp 1999 Jan; 60: 1–14
Puig JG, Mateos F, Buño A, et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens 1999 Jul; 17: 1033–9
Ilson BE, Martin DE, Boike SC, et al. The effects of eprosartan, an angiotensin II AT1 receptor antagonist, on uric acid excretion in patients with mild to moderate essential hypertension. J Clin Pharmacol 1998 May; 38: 437–41
Levine B, Eprosartan Multinational Study Group. Effect of eprosartan and enalapril in the treatment of black hypertensive patients: subgroup analysis of a 26-week, double-blind, multicentre study. Curr Med Res Opin 1999; 15(1): 25–32
Argenziano L, Trimarco B, Eprosartan Multinational Study Group. Effect of eprosartan and enalapril in the treatment of elderly hypertensive patients: subgroup analysis of a 26-week, double-blind, multicentre study. Curr Med Res Opin 1999; 15(1): 9–14
Gavras I, Gavras H. Safety and tolerability of eprosartan. Pharmacotherapy 1999 Apr; 19 (Pt 2): 102–7
Harland D, Duff D, Laing S, et al. Safety of eprosartan in elderly patients with hypertension. Am J Hypertens 1998 Apr; 11 Pt 2: 78
Town GI, Hallwright GP, Maling TJ, et al. Angiotensin converting enzyme inhibitors and cough. N Engl J Med 1987; 300: 161–3
Ravid D, Lishner M, Lang R, et al. Angiotensin-converting enzyme inhibitors and cough: a prospective evaluation in hypertension and in congestive heart failure. J Clin Pharmacol 1994; 34: 1116–20
Yeo WW, Ramsay LE. Persistent drug cough with enalapril: incidence depends on method used. J Hum Hypertens 1990; 4: 517–20
Simon SR, Moser M, Black HR, et al. Cough and ACE-inhibitors. Arch Intern Med 1992; 152: 1698–700
Sixth Report of the Joint National Committee on the Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Arch Intern Med 1997; 157: 2413–46
Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet 1999; 354: 1751–6
Girvin B, Johnston GD. The implications of noncompliance with antihypertensive medication. Drugs 1999; 52(2): 186–95
Hawkins DW, Bussey HI, Prisant LM. Hypertension. In: DiPiro JT, Talbert RL, Hayes PE, et al., editors. Pharmacotherapy: a pathophysiologic approach. 2nd edition ed. Norwalk (CT): Appleton & Lange, 1993: 139–59
Gress TW, Nieto FJ, Shahar E, et al. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. N Engl J Med 2000; 342: 905–12
BurnierM, BrunnerHR. Comparative antihypertensive effects of angiotensin II receptor antagonists. J Am Soc Nephrol 1999 Apr; 10 Suppl.: S278–82
Himmelmann A. Eprosartan. Drugs 1998 May; 55: 719
Smulyan H, Safar ME. The diastolic blood pressure in systolic hypertension. Ann Intern Med 2000; 132: 233–7
Black HR. Isolated systolic hypertension in the elderly: lessons from clinical trials and future directions. J Hypertens 1999; 17 Suppl. 5: S49–54
SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265(24): 3255–64
Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350: 757–64
Wang JG, Staessen JA, Gong L, et al. Chinese trial on isolated systolic hypertension in the elderly. Systolic Hypertension in China (Syst-China) Collaborative Group. Arch Intern Med 2000; 160(2): 211–20
UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317: 703–13
Hypertension in Diabetes Study Group. Hypertension in diabetes study IV. Therapeutic requirements to maintain tight blood pressure control. Diabetologia 1996; 39: 1554–61
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: I. Gavras, Hypertension and Atherosclerosis Section, Boston University School of Medicine, Boston, Massachusetts, USA; A.H. Gradman, Division of Cardiovascular Diseases, Western Pennsylvania Hospital, Pittsburgh, Pennsylvania, USA; G.D. Johnston, Department of Therapeutics and Pharmacology, Queen’s University of Belfast, Belfast, Northern Ireland; B. Levine, Department of Medicine, VA Medical Center, Los Angeles, California, USA; B.N.C. Prichard, Centre for Clinical Pharmacology, University College, London, England; M. Ravid, Department of Medicine, Meir Hospital, Kfar-Sava, Israel; D.A. Sica, Department of Clinical Pharmacology and Hypertension, Medical College of Virginia, Virginia Commonwealth University, Richmond, Virginia, USA.
Data Selection
Sources: Medical literature published in any language since 1983 on eprosartan, identified using AdisBase (a proprietary database of Adis International, Auckland, New Zealand), Medline and EMBASE. Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: AdisBase search terms were ‘Eprosartan’ or ‘SKF-108566’. Medline search terms were ‘Eprosartan’ or ‘SKF-108566’. EMBASE search terms were ‘Eprosartan’ or ‘SKF-108566’. Searches were last updated 20 June 2000.
Selection: Studies in patients with hypertension who received eprosartan. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Eprosartan, hypertension, antihypertensive, pharmacodynamics, pharmacokinetics, therapeutic use, drug interactions, tolerability.
Rights and permissions
About this article
Cite this article
Plosker, G.L., Foster, R.H. Eprosartan. Drugs 60, 177–201 (2000). https://doi.org/10.2165/00003495-200060010-00009
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200060010-00009