Abstract
-
▴ Alosetron is a potent and highly selective serotonin 5-HT3 receptor antagonist which has been evaluated for the management of irritable bowel syndrome (IBS). It blocked the fast 5HT3-mediated depolarisation of guinea-pig myenteric and submucosal neurons in vitro, with half-maximal inhibition at approximately 55 nmol/L.
-
▴ Alosetron attenuated the visceral nociceptive effect of rectal distension in conscious or anaesthetised dogs. It increased the compliance of the colon to distension in patients with IBS and delayed colonic transit in patients with IBS or carcinoid diarrhoea and in healthy volunteers.
-
▴ A single dose of alosetron 4mg increased in vivo fluid absorption in normal human small intestine.
-
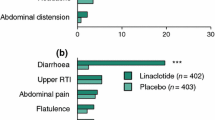
▴ In clinical trials in patients with IBS, alosetron 1mg twice daily was effective in relieving abdominal pain and discomfort. Alosetron was most effective in female patients and particularly in those with diarrhoea-predominant IBS.
-
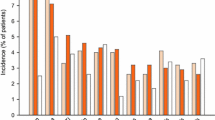
▴ In patients with IBS and healthy volunteers who received alosetron, the most common adverse event was constipation.


Similar content being viewed by others
References
Baxendale A, Bountra C, Clayton N, et al. Irritable bowel syndrome as visceral hyperalgesia: implications for therapy? Current Opinion in Central and Peripheral Nervous System Investigational Drugs 1999; 1(1): 86–97
Drossman DA, Whitehead WE, Camilleri M, et al. Irritable bowel syndrome: a technical review for practice guideline development. Gastroenterology 1997 Jun; 112: 2120–37
Kilpatrick GJ, Hagan RM, Butler A, et al. GR68755, a potent and selective antagonist of 5-HT3 receptors [abstract]. Br J Pharmacol 1991 Oct; 104 Suppl.: 259P
Kilpatrick GJ, Hagan RM, Oxford AW, et al. GR-68755 hydrochloride. Drugs Future 1992; 17(8): 660–4
Farthing MJG. New drugs in the management of the irritable bowel syndrome. Drugs 1998 Jul; 56: 11–21
Gershon MD. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther 1999; 13 Suppl. 2: 15–20
Pan H, Gershon MD. Effects of alosetron on the activation of submucosal primary afferent neurons, the peristaltic reflex, and responses of enteric neurons to 5-HT [abstract]. Gastroenterology 1999 Apr; 116 (Pt 2): 1058
Corrigan B, Lettis S, Kersey K, et al. Concentration effect relationship between alosetron and intradermal 5-HT induced flare response in volunteers following oral dosing [abstract]. PharmSci 1999; 1S: 2127
Humphrey PPA, Bountra C, Clayton N, et al. Review article: the therapeutic potential of 5-HT3 receptor antagonists in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 1999; 13 Suppl. 2: 31–8
Miura M, Lawson DC, Clary EM, et al. Central modulation of rectal distension-induced blood pressure changes by alosetron, a 5-HT3 receptor antagonist. Dig Dis Sci 1999 Jan; 44: 20–4
Delvaux M, Louvel D, Mamet J-R Effect of alosetron on responses to colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther 1998 Sep; 12: 849–55
Zerbib F, Bruley des Varannes, Oriola RC, et al. Alosetron does not affect the visceral perception of gastric distension in healthy subjects. Aliment Pharmacol Ther 1994 Aug; 8: 403–7
Bearcroft CP, André EA, Farthing MJG. In vivo effects of the 5-HT3 antagonist alosetron on basal and cholera toxin-induced secretion in the human jejunum: a segmental perfusion study. Aliment Pharmacol Ther 1997 Dec; 11: 1109–14
Foster JM, Houghton LA, Whorwell PJ. Alosetron slows colonic transit in patients with irritable bowel syndrome [abstract]. Gastroenterology 1997 Apr; 112 Suppl.: 732
Houghton LA, Foster J, Whorwell PJ, et al. Effect of alosetron, a new 5HT3 antagonist, on gastrointestinal transit in normal healthy volunteers [abstract]. Gastroenterology 1995 Apr; 108 Suppl.: 618
Tomlin J, McDonald JN, Read NW. The effect of a 5HT3 antagonist (alosetron) on the transit of a meal and the ileal brake in man [abstract]. Gastroenterology 1994 Apr; 106 Suppl.: 579
Saslow SB, Scolapio JS, Camilleri M, et al. Medium term effects of a new 5HT3 antagonist, alosetron, in patients with carcinoid diarrhoea. Gut 1998 May; 42: 628–34
Khoury R, Peghini P, Katz P, et al. Alosetron, a new 5HT3 antagonist has no adverse effects on esophageal sphincter (LES) pressure. Gastroenterology 1998; 114 (4, Pt 2): A732
Nomura T, Evans DF, Wingate DL, et al. Alosetron, a selective 5-HT3 antagonist, does not perturb normal human small bowel motor patterns [abstract]. Gastroenterology 1998 Apr 15 (Pt 2); 114 Suppl.: 811
Hsyu PH, Pritchard JF, Lloyd TL. Safety and age, gender, and time dependent pharmacokinetics of alosetron [abstract]. Pharm Res 1995 Sep; 12 Suppl.: 387
Corrigan B, Manzo J, James C, et al. The effect of repeat dosing on the pharmacokinetics of alosetron 1mg bid [abstract]. PharmSci 1998; 1: 5484
Bradbury A, Cozens SJ, Evans DC, et al. The disposition of GR 68755 after oral administration in man [abstract]. Br J Clin Pharmacol 1991 Nov; 32: 654
Andrew PD, Murkitt GS, Millson DS, et al. The determination of GR 68755 in plasma and its pharmacokinetic profile in human volunteers. Br J Clin Pharmacol 1991 Nov; 32: 667P
Gunput MD. Review article: clinical pharmacology of alosetron. Aliment Pharmacol Ther 1999; 13 Suppl. 2: 70–6
Corrigan B, Palmer J, Gillotin C, et al. The effect of food on the pharmacokinetics of alosetron [abstract]. PharmSci 1998; 1 S484
Gupta SK, Kunka RL, Metz A, et al. Effect of alosetron (a new 5-HT3 receptor antagonist) on the pharmacokinetics of haloperidol in schizophrenic patients. J Clin Pharmacol 1995 Feb; 35: 202–7
Palmer J, Noordin N, Andrew P, et al. The effect of gender on the pharmacokinetics of alosetron [abstract]. PharmSci 1998; 1: S465
Knaggs AR, Cable KM, Cannell RJP, et al. Biotransformation of alosetron: mechanism of hydantoin formation. Tetrahedron Lett 1995; 36(3): 477–80
D’Souza D, Jewell D, Ricci B, et al. Effect of alosetron (A) on the pharmacokinetics of cisapride (C), and examination of QT interval [abstract]. PharmSci 1999 Suppl. 1S: 2086
Hedayetullah N, Jewell D, Willmore E, et al. Effect of alosetron on pharmacokinetics (pk) of theophylline [abstract]. Pharm Sci 1999; IS: 2483
Manzo J, Simms L, Koch K, et al. Effect of alosetron on pharmacokinetics (pk) of levonorgestrel (LN) and ethinyl estradiol (EE) [abstract]. PharmSci 1999; 1S: 2080
Bardhan KD, Bodemar G, Geldof II, et al. A double-blind, randomized, placebo-controlled dose-ranging study to evaluate the efficacy of alosetron in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2000; 14: 23–34
Camilleri M, Mayer EA, Drossman DA, et al. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment Pharmacol Ther 1999; 13: 1149–59
Jones RH, Holtmann G, Rodrigo L, et al. Alosetron relieves pain and improves bowel function compared with mebeverine in female nonconstipated irritable bowel syndrome patients. Aliment Pharmacol Ther 1999; 13: 1419–27
Mangel AW, Hahn BA, Heath AT, et al. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res 1998; 26: 76–81
Mangel AW, Camilleri M, Chey WY, et al. Treatment of female IBS patients with alosetron. A potent and selective 5HT-3 receptor antagonist [abstract]. Gastroenterology 1999 Apr; 116 (Pt 2): 1036
Mangel AW, Camilleri M, Chey WY, et al. Alosetron, a 5-HT3 receptor antagonist, in the treatment of non-constipated female IBS patients [abstract]. Am J Gastroenterol 1999; 94(9): 2677
Lacey LA, McSorly D, Northcutt A, et al. Impact of alosetron on health related quality of life (HRQOL) and productivity in female patients with irritable bowel syndrome [abstract]. Gut 1999; 45 Suppl. V
Watson MW, Lacey LA, McSorley D, et al. Impact of alosetron on health-related quality of life (HRQOL) in female patients with irritable bowel syndrome (IBS) [abstract]. Am J Gastroenterol 1999; 94: 2762
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balfour, J.A.B., Goa, K.L. & Perry, C.M. Alosetron. Drugs 59, 511–518 (2000). https://doi.org/10.2165/00003495-200059030-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200059030-00008