Abstract
Background
Ramosetron is a potent and selective serotonin type 3 receptor antagonist. This meta-analysis aimed to analyze the efficacy and safety of ramosetron for irritable bowel syndrome with diarrhea (IBS-D).
Methods
Pubmed, MEDLINE, EMBASE and the Cochrane Library were searched for randomized controlled trials investigating the efficacy and safety of ramosetron for IBS-D. Risk of bias was assessed as described in the Cochrane handbook. A random effects model was used to calculate the effects of ramosetron vs placebo on symptomatic improvements, including relief of overall IBS symptoms, relief of abdominal discomfort/pain, improvement in abnormal bowel habits, and improvement in stool consistency, expressed as pooled relative risks (RRs) with 95% confidence interval (CI). Adverse events data were also summarized with RRs.
Results
Four randomized controlled trials involving 1623 participants were included. Compared with placebo, ramosetron could lead to relief of overall IBS symptoms (RR 1.70; 95%CI 1.48, 1.95), relief of abdominal discomfort/pain (RR 1.41; 95%CI, 1.24, 1.59), improvement in abnormal bowel habits (RR 1.72; 95%CI, 1.50, 1.98) and improvement in stool consistency (RR 1.71; 95%CI 1.40, 2.08). Ramosetron could lead to relief of overall IBS symptoms in both male and female patients (RR; 95%CI: 1.94; 1.58, 2.38 and 1.49; 1.25, 1.79). The RR (95%CI) for reported adverse events of ramosetron vs placebo was 1.10 (0.97, 1.26) across all studies. No serious adverse events (e.g., ischemic colitis) were reported. The incidences of hard stool and constipation were higher in ramosetron group compared with placebo group (RR; 95%CI: 4.74; 3.00, 7.51 and 2.53; 1.57, 4.10, respectively).
Conclusions
Ramosetron had beneficial effects to both male and female IBS-D patients. Treatment with ramosetron could cause more hard stool and constipation, without severe adverse events.
Similar content being viewed by others
Background
Irritable bowel syndrome (IBS) is characterized as abdominal pain and/or discomfort with altered bowel habits, without any demonstrable structural and biochemical abnormalities [1]. Based on the predominant bowel habit, Rome III criteria categorize IBS into four subtypes: IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), mixed IBS (IBS-M), and unsubtyped IBS. IBS affects an estimated 10-15% of adults worldwide [2,3,4,5]. IBS leads to significant reduction in patients’ daily activities and quality of life [3, 6, 7], and accounts for a considerable amount of medical resources [8,9,10]. Therefore, development of effective, well-tolerated and safe IBS treatments is important for the patients, health-care systems, and society as a whole.
Serotonin type 3 (5-HT3) receptor is one of the seven subtypes serotonin (5-HT) receptors. This receptor has been identified on the intestinal nerves, and a number of studies have suggested its role in the pathogenesis of IBS [11, 12]. Treatment targeted at 5-HT3 receptor has been the focus of numbers of recent studies. Clinical trials and meta-analysis have confirmed the efficacy of 5-HT3 receptor antagonist alosetron in the treatment of IBS-D patients [13,14,15,16,17]. However, some serious gastrointestinal events (e.g., ischemic colitis and severe constipation) have been reported with alosetron use [14, 18]. Therefore, alosetron is only available exclusively for women with severe, chronic IBS-D refractory to conventional therapy in the USA.
Ramosetron is a potent and selective 5-HT3 receptor antagonist. It has been used as an antiemetic for cancer patients in their chemotherapy and for the prevention of postoperative nausea and vomiting for many years [19,20,21]. Clinical trials have evaluated the efficacy and safety of ramosetron in IBS-D patients [22,23,24,25,26]. Though the results of these available clinical trials have been generally favorable, there is still no evidence for the efficacy of ramosetron for IBS-D based on the systematic review and meta-analysis. The efficacy of ramosetron on the treatment of both male and female IBS-D population, and the safety data of ramosetron, need to be clearly elucidated. Therefore, we performed this systematic review and meta-analysis of randomized, controlled trials (RCTs) comparing ramosetron with placebo in the treatment of IBS-D, in order to obtain more accurate and comprehensive results to systematically evaluate the efficacy and safety of ramosetron in the treatment of abdominal symptoms and bowel function in IBS-D.
Methods
This meta-analysis was conducted according to the Cochrane collaboration’s systematic review Methodology [27] and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [28].
Search strategy
Pubmed, MEDLINE, EMBASE and the Cochrane Library were systematically searched from inception to November 2015. The following search terms were used: “IBS”, “irritable bowel syndrome” AND “ramosetron”, “ramosetron hydrochloride”. No language or document type restrictions were applied. All bibliographies of the identified relevant studies were also checked to identify any additional studies. After scanning titles and abstracts of articles selected from the initial search, we read through the full text of eligible articles. Two reviewers independently performed the search process and disagreements were resolved by discussion.
Study selection
Studies meeting the following criteria were included: adults patients (age above 18 years) with IBS-D diagnosed by Rome criteria; randomized, double-blind, placebo-controlled trials that compared the efficacy of ramosetron vs placebo; included dichotomous assessment of response to therapy in term of effect on global IBS symptoms, abdominal pain/discomfort or abnormal bowel habits. The exclusion criteria were as follows: no placebo group; review article; incomplete data; abstract later published for full-text article.
Outcome assessment
The primary measurements were the effects of ramosetron compared with placebo on symptomatic improvements, including relief of overall IBS symptoms, relief of abdominal discomfort/pain, improvement in abnormal bowel habits, and improvement in stool consistency. The secondary measurement was the adverse events occurring as a result of therapy, aiming to evaluate the safety of ramosetron.
Data extraction
All the data of the primary and secondary measurements were extracted as dichotomous outcomes based on criteria of treatment response defined by the authors of included studies. In addition, the following clinical data were extracted for each trial: country of origin, criteria used to define IBS, number of patients in each group, ramosetron dose, treatment duration, follow-up duration after therapy, and outcomes. Data were extracted as intention-to-treat analyses, with all dropouts assumed to be treatment failures, wherever trial reporting allowed this. All data were extracted independently by two investigators on to a Microsoft Excel spreadsheet. Disagreements were resolved after discussion with other investigators.
Risk of bias assessment
According to the Cochrane Handbook for Systematic Reviews of Interventions, risk of bias assessment tool could be used for study quality assessment of randomized control trials. Risk of bias was assessed as described in the Cochrane handbook [27]. This evaluates the random sequence generation, allocation concealment, whether blinding was implemented, the completeness of follow-up, whether there was evidence of selective reporting of outcomes, and other biases. Two review authors independently judged the risk of bias and disagreements were resolved by discussion.
Data synthesis and statistical analysis
All statistical analysis were performed using the Cochrane Collaboration software Review Manager (version 5.3, The Nordic Cochrane Centre, Copenhagen, Denmark). The effect of ramosetron in IBS was expressed as a relative risk (RR) with 95% confidence interval (CI) of symptomatic improvement with ramosetron compared with placebo, based on intention-to-treat analysis. Adverse events data were also summarized with RRs. The heterogeneity across the included studies was assessed using both the I2 statistic with a cutoff of ≥50% and the χ2-test with a P value <0.10. The pooled RRs with 95%CIs were calculated using a random effects model, in order to provide a more conservative estimate of the effect of ramosetron. The sensitivity analysis was performed by excluding each of the studies at a time and re-analyze the remaining studies. Given the small number of studies included in our analysis, we did not performed the statistical tests for assessing publication bias [29].
Results
Descriptions of included studies
Figure 1 shows the flow chart of study selection process. A total of 173 studies were identified in initial literature search. Based on the inclusion and exclusion criteria, 164 studies were excluded after screening the titles and abstracts. The full texts of the remaining 9 studies were thoroughly reviewed, and another 5 studies were excluded due to the following reasons: no placebo arm (n = 1), review article (n = 1), abstracts later published as full-text articles (n = 2) and incomplete data (n = 1). Finally, four RCTs of ramosetron vs placebo in IBS-D were included in the final meta-analysis [22, 23, 25, 26]. In this section of our study the coefficient of agreement between the two authors performing the study search was 1.0, which meant that there was no disagreement between the two authors. The reason for this was that both authors strictly followed the search criteria we established in this section. They used the same search terms in the same databases with no language or document type restrictions. All bibliographies of the identified relevant studies were also checked carefully to identify any additional studies, and both authors read through the full text of eligible articles after scanning titles and abstracts of articles selected from the initial search.
In the four RCTs, a total of 1623 participants (812 in ramosetron group and 811 in placebo group) were included. The clinical characteristics of included studies are listed in Table 1. All four studies were conducted in Japan. Two studies defined IBS-D based on Rome II criteria [25, 26], and another two studies used Rome III criteria [22, 23]. The treatment duration in all studies were 12 weeks, and the follow-up duration were also 12 weeks. All included studies used the same definition for the improvement of IBS symptoms.
Figure 2 shows the risk of bias for all studies assessed using the Cochrane Collaboration tool. Two studies did not describe the detail of sequence generation process, leading to unclear risk of bias in the domain of “random sequence generation”. All four studies did not describe the method of concealment in sufficient detail, leading to unclear risk of bias in the domain of “allocation concealment”. All studies reported the mainly expected primary and secondary outcomes. However, in three studies some detailed changes of symptoms were not reported. We defined these studies as unclear risk of bias in the domain “selective reporting”. Low risk of bias was seen for all studies in the domains of “blinding of participants and personnel”, “blinding of outcome” and “incomplete outcome data”. There was no other bias.
Efficacy of Ramosetron in the treatment of IBS-D
Effect on relief of overall IBS symptoms
All four included studies evaluated the effect of ramosetron on relief of overall IBS symptoms. A total of 812 patients were assigned to the ramosetron group, whereas 811 patients were assigned to the placebo group. As shown in Fig. 3a, the RR of relief of overall IBS symptoms after treatment with ramosetron vs placebo was 1.70 (95%CI 1.48, 1.95). No heterogeneity was detected across all studies (P = 0.47, I2 = 0%).
For the outcome of relief of overall IBS symptoms, the RR was 1.94 (95%CI 1.58, 2.38) for male, and 1.49 (95%CI 1.25, 1.79) for female, indicating that treatment with ramosetron could lead to significant relief of overall IBS symptoms for both male and female patients. (Fig. 4).
Effect on relief of abdominal discomfort/pain
All studies assessed the relief of abdominal discomfort/pain. Ramosetron resulted in a pooled RR of response of 1.41 (95% CI, 1.24, 1.59) compared with placebo. There was no heterogeneity across all studies (P = 0.82, I2 = 0%). (Fig. 3b).
Effect on improvement in abnormal bowel habits
All four studies evaluated the effect of ramosetron on improvement in abnormal bowel habits. The pooled RR (95% CI) for improvement in abnormal bowel habits was 1.72 (1.50, 1.98). There was no heterogeneity across all studies (P = 0.40, I2 = 0%). (Fig. 3c).
Effect on improvement in stool consistency
Two of the four studies reported the outcome of improvement in stool consistency [22, 23]. Ramosetron showed significant improvement in stool consistency compared with placebo (RR 1.71; 95% CI 1.40, 2.08). No heterogeneity was observed (P = 0.80, I2 = 0.0%). (Fig. 3d).
Sensitivity analysis
Due to the difference in the sample size of the component RCTs, a sensitivity analysis was performed by excluding each of the studies at a time and re-analyze the remaining. The results of sensitivity analysis in this study are shown in Table 2. Although the relative risk changed, the overall conclusions were still the same, which meant that none of the studies had an undue influence on the final relative risk.
Safety
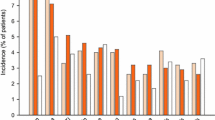
As shown in Fig. 5, safety profile was evaluated in all the included studies. In total, 54.9% of patients assigned to ramosetron experienced adverse events, compared with 49.0% of patients allocated to placebo. The RR (95%CI) for reported adverse events of ramosetron in comparison with placebo was 1.10 (0.97, 1.26) across all studies. There were no serious adverse events, especially no cases of ischemic colitis and severe constipation were reported. The most common adverse events with usage of ramosetron were hard stool and constipation. When data were pooled, the incidence of hard stool and constipation were both significantly higher in patients randomized to ramosetron than those receiving placebo, as the RRs (95% CI) were 4.74 (3.00, 7.51) and 2.53 (1.57, 4.10), respectively.
Discussion
This systematic review and meta-analysis confirmed the benefits of ramosetron over placebo in patients with IBS-D. Treatment with ramosetron was associated with symptomatic improvements, including relief of overall IBS symptoms, relief of abdominal discomfort/pain, improvement in abnormal bowel habits, and improvement in stool consistency. Treatment with ramosetron showed efficacy on both male and female patients. Hard stool and constipation occurred more frequently in patients on ramosetron compared with placebo. However, ramosetron is considered to be safe and well tolerated, because no severe adverse events were reported and the incidence of total adverse events was similar in ramosetron and placebo arm. Its clear efficacy and low incidence of serious adverse events suggest that ramosetron is a promising therapeutic agent for IBS-D patients.
Although great progress has been achieved in the understanding of IBS, its exact mechanism remains obscure and currently conventional treatments remain unsatisfied. As summarized by American College of Gastroenterology, the quality of evidence for a lot of currently available managements for IBS, such as dietary manipulation, certain antispasmodics, peppermint oil, non-absorbable antibiotics, etc. were graded as “very low”, “low” or “moderate”. Therefore, we still lack sufficiently effective treatments for IBS [30]. It is certainly of significance to provide strong evidence for the novel drugs used for IBS. Although the results of previously trials have been generally favorable, our present study provides an evidence of systematic review and meta-analysis for the efficacy and safety of ramosetron for IBS-D. What is more, the similarity across all eligible trials, including enrollment criteria (Rome criteria), design (randomized, double-blind, placebo-controlled), outcome measurements (same outcomes and same definition of outcomes), and treatment and follow-up duration (12 weeks), results in high comparability of our results and makes the pooled estimates robust and convincing.
In consideration of the involvement of 5-HT and the 5-HT3 receptors in the pathogenesis of IBS, a number of clinical trials have been conducted to evaluate the efficacy of 5-HT3 antagonists. Most of these trials were of good quality, but it was noteworthy that these kinds of drugs were associated with a potential risk of ischemic colitis and cardiovascular events respectively [18]. These risks largely limit the widely clinical application of these drugs. For instance, alosetron is only available exclusively for women with severe, chronic IBS-D refractory to conventional therapy in the USA. In a recently published open-label, uncontrolled, long-term safety trial, constipation occurred in 19.7% of IBS-D patients receiving 2.5 μg and 10.5% of patients receiving 5 μg of ramosetron, and there was no serious adverse event related to ramosetron, especially ischemic colitis [31]. In our meta-analysis, there was no significant difference in the incidence of total adverse events in ramosetron group and placebo group (RR 1.10; 95%CI 0.97, 1.26). Ramosetron did lead to more hard stool and constipation, which are considered to be the classic effect of 5-HT3 receptor antagonist. However, there were no severe adverse events, especially no case of ischemic colitis were reported. In addition, the incidence of constipation due to administration of ramosetron was only 7.02%, which was much lower than that in alosetron group (23.9%) reported previously [14]. This is extremely important due to the possible relationship between the ischemic colitis and constipation [32]. Lower incidence of constipation in ramosetron than in alosetron means ramosetron is less likely cause ischemic colitis. All these data demonstrate that ramosetron is not only effective, but also safe for the treatment of IBS-D patients.
The 5-HT3 receptor antagonist alosetron has been approved for only female patients with IBS-D, since the efficacy of alosetron has not been confirmed in males [33]. Development of effective drugs for male IBS-D patients is therefore in urgent need. The efficacy of ramosetron has been evaluated in both male and female populations in previous trials [22, 23, 25, 26]. In our meta-analysis, we pooled the dichotomous data of relief of overall IBS symptom. For both male and female patients, ramosetron could lead to significant relief of overall IBS symptoms (RR 1.94; 95%CI 1.58, 2.38 vs RR 1.49; 95%CI 1.25, 1.79. respectively). These pooled data revealed that ramosetron should be recommended for both male and female IBS-D patient in clinical practice. Many possible factors, including sexual cycle, biobehavioral responses to stress, differences in roles and emotions between males and females, may affect gender differences in the response to treatments with alosetron and ramosetron [34]. Furthermore, alosetron could transfer to the brain while ramosetron acts only on peripheral tissues, which may also contribute to the gender differences in response to different 5-HT3 receptor antagonists [26]. The exact mechanism of gender differences in the treatment response need to be further investigated.
Although most outcomes in the included studies are subjective and were measured on an ordinal scale, and were analyzed as dichotomous outcomes in the meta-analysis, the definitions for symptom relief were consistent across studies. In all included studies, the monthly responder was defined as a patient who had experienced “Completely relieved” or “Considerably relieved” for at least 2 weeks of the 4-week treatment, and the monthly responder rate of “symptom relief in IBS” was analyzed in these studies. What is more, in these different studies subjective outcomes were measured using standardized and validated tools. Severity of abdominal pain and discomfort was assessed daily on a 5-point scale (0: none, 1: mild, 2: moderate, 3: severe, 4: intolerable), and stool form data was scored on a 7-point ordinal scale according to the Bristol Stool Form Scale. Above consistent definitions for symptom relief and standardized and validated tools used for subjective outcomes measurement all make the pooled estimates in our meta-analysis robust and convincing.
There are several limitations in our present meta-analysis. First, the absolute number of clinical trials included is small. Although this might be partly compensated by the relative large amount of participants enrolled in each trial, further clinical trials are necessary to establish a more powerful evidence for the use of ramosetron in IBS-D. We did not evaluate the publication bias, also due to insufficient (less than 10) eligible studies included in our analysis, in line with recent recommendations [29]. Second, all of the included trials are conducted in Japan. This could limit the generalizability of the results to broader populations worldwide. The efficacy and safety of another 5-HT3 receptor antagonist alosetron have been demonstrated in Western populations [13, 15]. The efficacy and safety data of ramosetron for the treatment of IBS-D in Western population are essential and in urgent need. Third, all the included trials had high quality, however, the blinding methods used in these trials are not sufficiently described, especially for the allocation concealment. Further better designed randomized, placebo-controlled trials optimized to reduce bias are needed to reveal the efficacy of ramosetron in a broader population worldwide.
Conclusions
In conclusion, the significant pooled effect estimates in symptomatic improvements in ramosetron compared with placebo, combined with a good safety and tolerability profile, suggest that ramosetron is a significant development in the treatment of IBS-D. Treatment with ramosetron has similar efficacy for male and female patients. The efficacy of the drug in a broader population, especially in Western population, deserves further investigation in better designed clinical trials.
Abbreviations
- 5-HT:
-
Serotonin
- 5-HT3:
-
Serotonin type 3
- CI:
-
Confidence interval
- IBS:
-
Irritable bowel syndrome
- IBS-C:
-
Irritable bowel syndrome with constipation
- IBS-D:
-
Irritable bowel syndrome with diarrhea
- IBS-M:
-
Mixed irritable bowel syndrome
- RCTs:
-
Randomized, controlled trials
- RR:
-
Relative risk
References
American College of Gastroenterology Task Force on Irritable Bowel S, Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(Suppl 1):S1–35.
Saito YA, Schoenfeld P, Locke GR 3rd. The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97(8):1910–5.
Hungin AP, Chang L, Locke GR, Dennis EH, Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther. 2005;21(11):1365–75.
Quigley EM, Bytzer P, Jones R, Mearin F. Irritable bowel syndrome: the burden and unmet needs in Europe. Dig Liver Dis. 2006;38(10):717–23.
Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(7):712–21. e4
Dean BB, Aguilar D, Barghout V, Kahler KH, Frech F, Groves D, et al. Impairment in work productivity and health-related quality of life in patients with IBS. Am J Manag Care. 2005;11(1 Suppl):S17–26.
El-Serag HB, Olden K, Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther. 2002;16(6):1171–85.
Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136(2):376–86.
Camilleri M. Management of the irritable bowel syndrome. Gastroenterology. 2001;120(3):652–68.
Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology. 1995;109(6):1736–41.
Hirata T, Keto Y, Nakata M, Takeuchi A, Funatsu T, Akuzawa S, et al. Effects of serotonin 5-HT3 receptor antagonists on stress-induced colonic hyperalgesia and diarrhoea in rats: a comparative study with opioid receptor agonists, a muscarinic receptor antagonist and a synthetic polymer. Neurogastroenterol Motil. 2008;20(5):557–65.
Humphrey PP, Bountra C, Clayton N, Kozlowski K. Review article: the therapeutic potential of 5-HT3 receptor antagonists in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 1999;13(Suppl 2):31–8.
Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355(9209):1035–40.
Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6(5):545–55.
Cremonini F, Nicandro JP, Atkinson V, Shringarpure R, Chuang E, Lembo A. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Aliment Pharmacol Ther. 2012;36(5):437–48.
Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil. 2003;15(1):79–86.
Jones RH, Holtmann G, Rodrigo L, Ehsanullah RS, Crompton PM, Jacques LA, et al. Alosetron relieves pain and improves bowel function compared with mebeverine in female nonconstipated irritable bowel syndrome patients. Aliment Pharmacol Ther. 1999;13(11):1419–27.
Friedel D, Thomas R, Fisher RS. Ischemic colitis during treatment with alosetron. Gastroenterology. 2001;120(2):557–60.
Fujii Y, Saitoh Y, Tanaka H, Toyooka H. Ramosetron vs granisetron for the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Can J Anaesth. 1999;46(10):991–3.
Shi Y, He X, Yang S, Ai B, Zhang C, Huang D, et al. Ramosetron versus ondansetron in the prevention of chemotherapy-induced gastrointestinal side effects: a prospective randomized controlled study. Chemotherapy. 2007;53(1):44–50.
Kim HJ, Shin SW, Song EK, Lee NR, Kim JS, Ahn JS, et al. Ramosetron versus Ondansetron in combination with Aprepitant and Dexamethasone for the prevention of highly Emetogenic chemotherapy-induced nausea and vomiting: a multicenter, randomized phase III trial, KCSG PC10-21. Oncologist. 2015;20(12):1440–7.
Fukudo S, Ida M, Akiho H, Nakashima Y, Matsueda K. Effect of ramosetron on stool consistency in male patients with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2014;12(6):953–9. e4
Fukudo S, Kinoshita Y, Okumura T, Ida M, Akiho H, Nakashima Y, et al. Ramosetron reduces symptoms of irritable bowel syndrome with Diarrhea and improves quality of life in women. Gastroenterology. 2016;150(2):358–66. e8
Lee KJ, Kim NY, Kwon JK, Huh KC, Lee OY, Lee JS, et al. Efficacy of ramosetron in the treatment of male patients with irritable bowel syndrome with diarrhea: a multicenter, randomized clinical trial, compared with mebeverine. Neurogastroenterol Motil. 2011;23(12):1098–104.
Matsueda K, Harasawa S, Hongo M, Hiwatashi N, Sasaki D. A randomized, double-blind, placebo-controlled clinical trial of the effectiveness of the novel serotonin type 3 receptor antagonist ramosetron in both male and female Japanese patients with diarrhea-predominant irritable bowel syndrome. Scand J Gastroenterol. 2008;43(10):1202–11.
Matsueda K, Harasawa S, Hongo M, Hiwatashi N, Sasaki D. A phase II trial of the novel serotonin type 3 receptor antagonist ramosetron in Japanese male and female patients with diarrhea-predominant irritable bowel syndrome. Digestion. 2008;77(3-4):225–35.
Higgins JPT, Sally Green. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://handbook-5-1.cochrane.org/.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002.
Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(Suppl 1):S2–26. quiz S7
Fukudo S, Kinoshita Y, Okumura T, Ida M, Hayashi K, Akiho H, et al. Effect of ramosetron in female patients with irritable bowel syndrome with diarrhea: a phase III long-term study. J Gastroenterol. 2016;51(9):874–82.
Suh DC, Kahler KH, Choi IS, Shin H, Kralstein J, Shetzline M. Patients with irritable bowel syndrome or constipation have an increased risk for ischaemic colitis. Aliment Pharmacol Ther. 2007;25(6):681–92.
Camilleri M, Mayer EA, Drossman DA, Heath A, Dukes GE, McSorley D, et al. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment Pharmacol Ther. 1999;13(9):1149–59.
Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123(5):1686–701.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (81330012 and 81370495).
Availability of data and materials
All data generated or analysed during this study are included in this article.
Author information
Authors and Affiliations
Contributions
QQQ and YQL designed the research; QQQ, YZ and FXC performed the research and analyzed the data; QQQ wrote the manuscript; XLZ and YQL reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Authors’ information
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Qi, Q., Zhang, Y., Chen, F. et al. Ramosetron for the treatment of irritable bowel syndrome with diarrhea: a systematic review and meta-analysis of randomized controlled trials. BMC Gastroenterol 18, 5 (2018). https://doi.org/10.1186/s12876-017-0734-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-017-0734-2