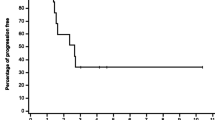
Abstract
Extended schedules of oral etoposide have been evaluated in many types of advanced cancer. In addition to their use in the common solid tumours, extended schedules have been employed in Kaposi’s sarcoma (both AIDS-related and endemic types), medulloblastoma, glioma, and hepatocellular carcinoma. Single agent activity was demonstrated in all of these tumour subtypes. For patients with carcinoma of unknown primary site, we have recently incorporated a 10-day oral etoposide schedule into a combination regimen that also includes paclitaxel and carboplatin. With this regimen we achieved a 47% response rate in a group of 53 evaluable patients, with a median survival of 13.4 months. Patients with adenocarcinoma and poorly differentiated carcinoma of unknown primary site had comparable response rates and survival. According to a large number of clinical trials and pharmacokinetic data, a daily oral etoposide dose of 50 mg/m2 consistently produces serum concentrations >1 mg/L for several hours each day. Lower doses fail to consistently produce this serum concentration, which is considered necessary for optimum tumoricidal activity. Optimal dose duration is 10 to 14 days, particularly when combination regimens are being employed. Oral etoposide has an established role as a single agent in patients with low grade non-Hodgkin’s lymphoma, Kaposi’s sarcoma, and testicular cancer (if residual carcinoma is resected after first-line treatment). The optimal use of extended-schedule etoposide in combination regimens is not defined but is being evaluated in a number of etoposide-sensitive malignancies.
Similar content being viewed by others
References
Schwartsmann G, Sprinz E, Kromfield M, et al. Clinical and pharmacokinetic study of oral etoposide in patients with AIDS-related Kaposi’s sarcoma with no prior exposure to cytotoxic therapy. J Clin Oncol 1997; 15: 2218–24
Brambilla L, Labianca R, Boneschi V, et al. Mediterranean Kaposi’s sarcoma in the elderly: a randomized study of oral etoposide versus vinblastine. Cancer 1994; 74: 2873–8
Ashley DM, Meier L, Kerby T, et al. Response of recurrent medulloblastoma to low-dose oral etoposide. J Clin Oncol 1996; 14: 1922–7
Chamberlain MC, Grafe MR. Recurrent chiasmatic-hypothalamic glioma treated with oral etoposide. J Clin Oncol 1995; 13: 2072–6
Wierzbicki R, Ezzat A, Abdel-Warith A, et al. Phase II trial of chronic daily VP-16 administration in unresectable hepatocellular carcinoma (HCC). Ann Oncol 1994; 5: 466–7
Cheng AL, Chen YC, Yeh KH, et al. Chronic oral etoposide and tamoxifen in the treatment of far-advanced hepatocellular carcinoma. Cancer 1996; 77: 872–7
Hainsworth JD, Erland JB, Kaiman LA, et al. Carcinoma of unknown primary site: treatment with 1-hour paclitaxel, carboplatin, and extended schedule etoposide. J Clin Oncol 1997; 15: 2385–94
Johnson DH, Greco FA, Strupp J, et al. Prolonged administration of oral etoposide in patients with relapsed or refractory small cell lung cancer: a phase II trial. J Clin Oncol 1990; 8: 1613–7
Rose PG, Blessing JA, Mayer AR, et al. Prolonged oral etoposide as second-line therapy for platinum-resistant and platinum-sensitive ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 1998; 16: 405–10
Martin M, Lluch A, Casado A, et al. Clinical activity of chronic oral etoposide in previously treated metastatic breast cancer. J Clin Oncol 1994; 12: 986–91
Hainsworth JD, Johnson DH, Frazier SR, et al. Chronic daily administration of oral etoposide in refractory lymphoma. Eur J Cancer 1990; 26: 818–21
Miller JC, Einhorn LH. Phase II study of daily oral etoposide in refractory germ cell tumors. Semin Oncol 1990; 2 Suppl. 17: 36–9
Dombernowsky P, Nissen NI. Schedule dependency of the antileukemic activity of the podophyllotoxin-derivative VP-16-213 (NSC-141540)inL12101eukemia. Acta Pathol Microbiol Scand Section A 1973; 81: 715–24
Rivera G, Avery T, Roberts DW. Response of L1210 to combinations of cytosine arabinoside and VM-26 or VP16-213. Eur J Cancer 1975; 11: 639–47
Stahelin H. Activity of a new glycosidic lignan derivative (VP16-213) related to podophyllotoxin in experimental tumors. Eur J Cancer 1973; 9: 215–21
D’Incalci M, Erba E, Vaghi M, et al. In vitro cytotoxicity of VP-16 on primary tumor and metastasis of Lewis lung carcinoma. Eur J Cancer Clin Oncol 1982; 18: 377–80
Brindley CJ, Pedley RB, Antoniw P, et al. Activity and distribution studies of etoposide and mitozolomide in vivo and in vitro against human choriocarcinoma cell lines. Cancer Chemother Pharmacol 1987; 19: 221–5
Slevin ML, Clark PI, Joel SP, et al. A randomized trial to evaluate the effect of schedule on the activity of etoposide in small cell lung cancer. J Clin Oncol 1989; 7: 1330–40
Hande KR, Krozely MG, Greco FA, et al. Bioavailability of low-dose oral etoposide. J Clin Oncol 1993; 11: 374–7
Clark PI, Cottier B. The activity of 10-, 14-, and 21-day schedules of single-agent etoposide in previously untreated patients with extensive small cell lung cancer. Semin Oncol 1992; 14 Suppl. 19: 36–9
Thompson DS, Hainsworth JD, Hande KR, et al. Prolonged administration of low-dose, infusional etoposide in patients with etoposide-sensitive neoplasms: a phase I/II study. J Clin Oncol 1993; 11: 1322–8
Hainsworth JD, Johnson DH, Frazier SR, et al. Chronic administration of oral etoposide —a phase I trial. J Clin Oncol 1989; 7: 396–401
Hainsworth JD, Gray JR, Stroup SL, et al. Paclitaxel, carboplatin, and extended schedule etoposide in the treatment of small cell lung cancer: comparison of sequential phase II trials using different dose intensities. J Clin Oncol 1997; 15: 3464–71
de Jong RS, Mulder NH, Uges DR, et al. Randomized comparison of oral pharmacokinetics after oral etoposide phosphate and oral etoposide. Br J Cancer 1997; 75: 1660–6
Cooper MA, Einhorn LH. Maintenance chemotherapy with daily oral etoposide following salvage therapy in patients with germ cell tumors. J Clin Oncol 1995; 13: 1167–9
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hainsworth, J.D. Extended-Schedule Oral Etoposide in Selected Neoplasms and Overview of Administration and Scheduling Issues. Drugs 58 (Suppl 3), 51–56 (1999). https://doi.org/10.2165/00003495-199958003-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199958003-00008