Summary
Synopsis
Incorporation of amphotericin B into small unilamellar liposomes (AmBisome® ) alters the pharmacokinetic properties of the drug, but allows it to retain significant in vitro and in vivo tactivity against fungal species, including Candida, Aspergillus and Cryptococcus, and parasites of the genus Leishmania.
Used as prophylaxis against fungal infections in immunocompromised patients, liposomal amphotericin B appeared to reduce the incidence of both fungal colonisation and proven fungal infections, but did not affect overall survival.
Empirical therapy with liposomal amphotericin B in immunocompromised adults or children with suspected fungal infections was at least as effective as therapy with conventional amphotericin B. In the largest noncomparative studies, liposomal amphotericin B produced mycological eradication in 40 and 83% of patients with proven Candida infections and 41 and 60% with proven Aspergillus infections; however, these studies included relatively few patients. Mycological eradication rates of 67 to 85% in patients with cryptococcal meningitis have been reported
Liposomal amphotericin B is an effective treatment for visceral leishmaniasis in immunocompetent adults and children, including those with severe or drug-resistant disease. The drug also produces good response rates in immunocompromised patients; however, relapse rates in these patients are high.
Liposomal amphotericin B is generally well tolerated. Few patients require discontinuation or dose reduction of the drug because of adverse events. The most frequently reported adverse events are hypokalaemia, nephrotoxicity and infusion-related reactions; however, these occur significantly less often after liposomal amphotericin B than after the conventional formulation of the drug.
The acquisition cost of liposomal amphotericin B is higher than that of conventional amphotericin B. Cost-effectiveness analyses did not clearly show an economic benefit for empirical liposomal amphotericin B antifungal therapy in adults; however, one model suggested that initial empirical therapy with the liposomal formulation in children may cost less per cure than initial therapy with the conventional formulation.
Liposomal amphotericin B appears to be an effective alternative to conventional amphotericin B in the management of immunocompromised patients with proven or suspected fungal infections. Use of the drug is facilitated by its greatly improved tolerability profile compared with conventional amphotericin B. Because of this, liposomal amphotericin should be preferred to conventional amphotericin B in the management of suspected or proven fungal infections in immunocompromised patients with pre-existing renal dysfunction, amphotericin B-induced toxicity or failure to respond to conventional amphotericin B. Liposomal amphotericin B may also be considered for first- or second-line treatment of immunocompetent patients with visceral leishmaniasis.
Pharmacodynamics
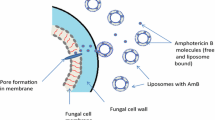
Amphotericin B is a macrocyclic polyene antibiotic which acts via inhibition of membrane function in susceptible fungal and Leishmania cells. Liposomal amphotericin B (AmBisome®) — produced by incorporation of amphotericin B into small, unilamellar liposomes — accumulates at sites of fungal infection, binding directly to fungal cells and causing cell death. Drug and liposome remain closely associated in circulation, permitting the administration of higher doses with reduced toxicity relative to conventional amphotericin B.
Liposomal amphotericin B is active in vitro and in vivo against a variety of pathogenic fungi and Leishmania species. In rodent models of Candida, Aspergillus and Cryptococcus infection, liposomal amphotericin B was administered in higher doses than the conventional drug, generally producing greater reductions in fungal burden. Survival rates were generally similar between the 2 formulations, although liposomal amphotericin B improved cure rates and survival compared with lower doses of conventional amphotericin B in some studies. When the 2 formulations were administered in identical milligram per kilogram dosages, they had similar effects on survival in infected animals, and conventional amphotericin B tended to produce a greater reduction in fungal burden. Against murine visceral leishmaniasis, liposomal amphotericin B was considerably more effective than conventional amphotericin B or meglumine antimonate, and it produced clinical improvement with significantly fewer doses than the latter agent.
Pharmacokinetics
After intravenous administration, liposomal amphotericin B achieves a higher peak serum concentration and larger area under the serum concentration-time curve than conventional amphotericin B. The drug appears to be taken up extensively by the reticuloendothelial system. High concentrations of drug are detected in liver and spleen, with lower concentrations found in brain, CSF, bone marrow, heart and lung.
The apparent mean half-life of liposomal amphotericin B is approximately 6 to 7 hours. Elimination of liposomal amphotericin B, like that of the conventional formulation, is poorly understood. No metabolites are known.
Therapeutic Use in Fungal Infections
Liposomal amphotericin B fungal prophylaxis was more effective than placebo in immunocompromised patients. The drug significantly reduced rates of invasive fungal infection in liver transplant recipients; among bone marrow transplant recipients, liposomal amphotericin B reduced the rate of fungal colonisation, but not invasive fungal infection. Overall survival was not affected.
Empirical therapy with liposomal amphotericin B in immunocompromised adults and children with suspected fungal infections was at least as effective as therapy with conventional amphotericin B in randomised studies. Evidence from noncomparative studies confirms the effectiveness of empirical liposomal amphotericin B in patients with suspected fungal infections, including patients who had experienced prior inefficacy or toxicity with conventional amphotericin B.
Limited data from noncomparative studies suggest that liposomal amphotericin B is effective against invasive Candida and Aspergillus infection and oral candidosis. However, fewer than 20 patients with each infection were evaluable in most studies. In the largest available studies, liposomal amphotericin B produced mycological eradication in 41 and 60% of patients with Aspergillus infection and 40 and 83% of patients infected by Candida spp. 67 to 85% of patients with AIDS and cryptococcal infection (primarily meningitis) who received liposomal amphotericin B achieved mycological eradication.
Therapeutic Use in Visceral Leishmaniasis
Liposomal amphotericin B is an effective treatment for visceral leishmaniasis (kala azar) in immunocompetent adults and children, clearing parasites in 100% of patients in several studies. The drug is also effective in patients with severe or pentavalent antimonial-resistant disease; however, response rates appear to be lower. In most immunocompetent patients, symptomatic improvement and objective response are detectable within 1 week of starting therapy. Liposomal amphotericin B also produces good response rates in immunocompromised patients; however, relapse rates are high. No comparative data are available.
Tolerability
Liposomal amphotericin B was generally well tolerated. The drug was discontinued because of adverse events in <5% of patients. Liposomal amphotericin B recipients experienced fewer adverse events than patients who received conventional amphotericin B. The most frequently reported adverse events in the liposomal amphotericin B group included hypokalaemia, nephrotoxicity and infusion-related fever and rigors. Increased serum liver enzymes have been noted in a substantial proportion of liposomal amphotericin B recipients. However, these patients may have been predisposed to elevations in liver enzymes by concomitant drugs or disease states; causality is unclear.
Dosage and Administration
Liposomal amphotericin B is administered as a single daily dose by slow intravenous infusion. Premedication is not required.
Used for empirical antifungal therapy in immunocompromised patients, the recommended (US) liposomal amphotericin B dosage is 3 mg/kg/day. Patients with proven systemic fungal infections should receive 3 to 5 mg/kg/day (US) or 1 to 3 mg/kg/day (UK). The optimum duration of antifungal therapy is not well defined. In neutropenic patients, treatment is generally continued until the recovery of neutrophil counts. A dosage of liposomal amphotericin B 1 mg/kg/day has been used for fungal prophylaxis after bone marrow or liver transplantation.
In immunocompetent patients with visceral leishmaniasis, liposomal amphotericin B 3 mg/kg should be administered on days 1 to 5, 14 and 21. In immunocompromised patients, the recommended dosage is 3 mg/kg/day on days 1 to 5 and 4 mg/kg/day on days 10, 17, 24, 31 and 38. Other regimens of 21 to 30 mg/kg administered over 10 to 21 days may also be appropriate. as]Pharmacoeconomic Implications of Liposomal Amphotericin B
The acquisition cost of liposomal amphotericin B is considerably higher than that of the conventional formulation. Because of this, the prophylactic use of liposomal amphotericin B may be difficult to justify. One cost-effectiveness analysis of empirical liposomal amphotericin B therapy suggested that savings associated with the reduced toxicity and improved efficacy of the liposomal formulation in immunocompromised adults with proven or suspected fungal infections were not enough to offset its increased acquisition cost compared with the conventional formulation. Another model suggested that initial empirical therapy with liposomal amphotericin B may cost less per complete cure than the conventional formulation in children, but not in adults. No pharmacoeconomic data are available for the use of liposomal amphotericin B in patients with visceral leishmaniasis.
Similar content being viewed by others
References
Richardson MD, Warnock DW. Fungal Infection: diagnosis and management. Oxford: Blackwell Scientific Publications, 1993
Viviani MA, Rizzardini G, Tortorano AM, et al. Lipid-based amphotericin B in the treatment of cryptococcosis. Infection 1994 Mar–Apr; 22: 137–42
de Marie S, Janknegt R, Bakker-Woudenberg IAJM. Clinical use of liposomal and lipid-complexed amphotericin B. J Antimicrob Chemother 1994 May; 33: 907–16
Berman JD. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis 1997; 24: 684–703
Proffitt RT, Adler-Moore J, Fujii G, et al. Stability and mode of action of Ambisome® (liposomal amphotericin B). J Control Release 1994 Jan; 28: 342–3
Pallister CJ, Johnson EM, Warnock DW, et al. In-vitro effects of liposome-encapsulated amphotericin B (AmBisome) and amphotericin B-deoxycholate (Fungizone) on the phagocytic and candidacidal function of human polymorphonuclear leucocytes. J Antimicrob Chemother 1992 Sep; 30: 313–20
Schindler JJ, Warren RP, Allen SD, et al. Immunological effects of amphotericin B and liposomal amphotericin B on splenocytes from immune-normal and immune-compromised mice. Antimicrob Agents Chemother 1993 Dec; 37: 2716–21
Working Party of the British Society for Antimicrobial Chemotherapy. Working Party report: antifungal drug susceptibility testing. J Antimicrob Chemother 1995; 36: 899–909
Cormican MG, Pfaller MA. Standardization of antifungal susceptibility testing. J Antimicrob Chemother 1996; 38: 561–78
Anaissie E, Paetznick V, Proffitt R, et al. Comparison of the in vitro antifungal activity of free and liposome-encapsulated amphotericin B. Eur J Clin Microbiol Infect Dis 1991 Aug; 10: 665–8
Karyotakis NC, Anaissie EJ. Efficacy of escalating doses of liposomal amphotericin B (AmBisome) against hematogenous Candida lusitaniae and Candida krusei infection in neutropenic mice. Antimicrob Agents Chemother 1994 Nov; 38: 2660–2
Karyotakis NC, Anaissie EJ, Hachem R, et al. Comparison of the efficacy of polyenes and triazoles against hematogenous Candida krusei infection in neutropenic mice. J Infect Dis 1993 Nov; 168: 1311–3
van Etten EW, van den Heuvel-de-Groot C, Bakker-Woudenberg IA. Efficacies of amphotericin B-desoxycholate (Fungizone), liposomal amphotericin B (AmBisome) and fluconazole in the treatment of systemic candidosis in immunocompetent and leucopenic mice. J Antimicrob Chemother 1993 Nov; 32: 723–39
Kretschmar M, Nichterlein T, Hof H. Ambisome is superior to amphotericin B in lipid suspensions for treatment of murine candidiasis [abstract]. 7th Eur Congr Clin Microbiol Infect Dis 1995: 155
Adler-Moore JP, Chiang S-M, Satorius A, et al. Treatment of murine candidosis and cryptococcosis with a unilamellar liposomal amphotericin B formulation (AmBisome). J Antimicrob Chemother 1991 Oct; 28 Suppl. B 63–71
Francis P, Lee JW, Hoffman A, et al. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar D-mannitol and serum galactomannan as markers of infection. J Infect Dis 1994 Feb; 169: 356–68
Leenders ACAP, de Marie S, ten Kate MT, et al. Liposomal amphotericin B (AmBisome) reduces dissemination of infection as compared with amphotericin B deoxycholate (Fungizone) in a rat model of pulmonary aspergillosis. J Antimicrob Chemother 1996 Aug; 38: 215–25
Vassiloyanakopoulos A, Boutsikakis J, Mylonakis EE, et al. Comparative therapeutic efficacy of two amphotericin B liposomal forms in aspergillus fumigatus (AF) experimental endocarditis [abstract]. Clin Infect Dis 1995 Sep; 21: 779
Albert MM, Stahl-Carroll TL, Luther MF, et al. Comparison of liposomal amphotericin B to amphotericin B for treatment of murine cryptococcal meningitis. J Mycol Med 1995; 5(1): 1–6
Clemons KV, Stevens DA. Comparison of a liposomal amphotericin B formulation (AmBisome) and deoxycholate amphotericin B (Fungizone) for the treatment of murine paracoccidioidomycosis. J Med Vet Mycol 1993; 31(5): 387–94
Clemons KV, Stevens DA. Therapeutic efficacy of a liposomal formulation of amphotericin B (AmBisome) against murine blastomycosis. J Antimicrob Chemother 1993 Sep; 32: 465–72
Albert MM, Adams K, Luther MJ, et al. Efficacy of AmBisome in murine coccidioidomycosis. J Med Vet Mycol 1994 Dec; 32: 467–71
Anaissie EJ, Hachem R, Karyotakis NC, et al. Comparative efficacies of amphotericin B, triazoles, and combination of both as experimental therapy for murine trichosporonosis. Antimicrob Agents Chemother 1994 Nov; 38: 2541–4
Graybill JR, Bocanegra R. Liposomal amphotericin B therapy of murine histoplasmosis. Antimicrob Agents Chemother 1995 Aug; 39: 1885–7
Gilbert BE. Liposomal aerosols in the management of pulmonary infections. J Aerosol Med 1996; 9(1): 111–22
Yardley V, Croft SL. Activity of liposomal amphotericin B against experimental cutaneous leishmaniasis. Antimicrob Agents Chemother 1997; 41(4): 752–6
Gangneux J-P, Sulahian A, Garin YJ-F, et al. Lipid formulations of amphotericin B in the treatment of experimental visceral leishmaniasis due to Leishmania infantum. Trans R Soc Trop Med Hyg 1996 Sep–Oct; 90: 574–7
Gradoni L, Davidson RN, Orsini S, et al. Activity of liposomal amphotericin B (AmBisome) against Leishmania infantum and tissue distribution in mice. J Drug Target 1993; 1(4): 311–6
Oliva G, Gradoni L, Ciaramella P, et al. Activity of liposomal amphotericin B (AmBisome) in dogs naturally infected with Leishmania infantum. J Antimicrob Chemother 1995 Dec; 36: 1013–9
Proffitt RT, Satorius A, Chiang S-M, et al. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J Antimicrob Chemother 1991 Oct; 28 Suppl. B: 49–61
Janknegt R, de Marie S, Bakker-Woudenberg IAJM, et al. Liposomal and lipid formulations of amphotericin B: clinical pharmacokinetics. Clin Pharmacokinet 1992 Oct; 23: 279–91
Heinemann V, Kähny B, Debus A, et al. Pharmacokinetics of liposomal amphotericin B (AmBisome) versus other lipid-based formulations. Bone Marrow Transplant 1994; 14 Suppl. 5: S8–9
Walsh TJ, Bekersky I, Yeldandi V, et al. Pharmacokinetics of AmBisome in persistently febrile neutropenic patients receiving empirical antifungal therapy [abstract no. A13]. 35th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC): 1995 Sep 17–20; 3
NeXstar Pharmaceuticals Inc. AmBisome(R) (amphotericin B) lipossome for injection: prescribing information. Deerfield, IL, USA, Aug 1997
Ringdén O, Meunier F, Tollemar J, et al. Efficacy of amphotericin B encapsulated in liposomes (Ambisome) in the treatment of invasive fungal infections in immunocompromised patients. J Antimicrob Chemother 1991 Oct; 28 Suppl. B: 73–82
Tollemar J, Ringden O, Tyden G. Liposomal amphotericin-B (Ambisome) treatment in solid organ and bone marrow transplant recipients. Efficacy and safety evaluation. Clin Transpl 1990 Jun; 4: 167–75
Lee JW, Amantea MA, Francis PA, et al. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (AmBisome®) in rabbits [abstract]. Antimicrob Agents Chemother 1994 Apr; 38: 713–8
Gonzalez C, Sein T, Bacher J, et al. Penetration of lipid formulations of amphotericin B into cerbrospinal fluid and brain tissue [abstract no. A-90]. 37th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC): 1997 Sep 28–Oct 1; Toronto, 19
Tomlin M, Priestley GS. Elimination of liposomal amphotericin by hemodiafiltration [letter]. Intensive Care Med 1995 Aug; 21: 699–700
Humphreys H, Oliver DA, Winter R, et al. Lipososmal amphotericin B and continuous venous-venous haemofiltration [letter]. J Antimicrob Chemother 1994 May; 33: 1070–1
Meunier F. Targeting fungi: a challenge. Am J Med 1995 Dec 29; 99 Suppl. 6A: 60S–7S
Tollemar J, Ringdén O, Andersson S, et al. Randomized double-blind study of liposomal amphotericin B (Ambisome) prophylaxis of invasive fungal infections in bone marrow transplant recipients. Bone Marrow Transplant 1993 Dec; 12: 577–82
Tollemar J, Höckerstedt K, Ericzon B-G, et al. Prophylaxis with liposomal amphotericin B (AmBisome) prevents fungal infections in liver transplant recipients: long term results of a randomized, placebo-controlled trial. Transplant Proc 1995 Feb; 27: 1195–8
Walsh T, Bodensteiner D, Hiemenz J, et al. A randomized, double-blind trial of AmBisome (liposomal amphotericin B) versus amphotericin B in the empirical treatment of persistently febrile neutropenic patients [abstract no. LM-90]. 37th Inter-science Conference on Antimicrobial Agents and Chemotherapy (ICAAC): 1997 Sep 28–Oct 1; Toronto, 381
Prentice HG, Hahn IM, Herbrecht R, et al. A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients. Br J Haematol 1997; 98: 711–8
Berenguer J, Muñoz P, Parras F, et al. Treatment of deep mycoses with liposomal amphotericin B. Eur J Clin Microbiol Infect Dis 1994 Jun; 13: 504–7
Böhme A, Hoelzer D. Liposomal amphotericin B as early empiric antimycotic therapy of pneumonia in granulocytopenic patients. Mycoses 1996; 39: 419–26
Heinemann V, Pyka S, Bosse D, et al. Liposomal amphotericin B (AmBisome): clinical safety and efficacy [abstract]. Onkologie 1995 Oct; 18 Suppl. 2: 198
Krüger W, Stockschläder M, Rüssmann B, et al. Experience with liposomal amphotericin-B in 60 patients undergoing high-dose therapy and bone marrow or peripheral blood stem cell transplantation. Br J Haematol 1995 Nov; 91: 684–90
Mills W, Chopra R, Linch DC, et al. Liposomal amphotericin B in the treatment of fungal infections in neutropenic patients: a single-centre experience of 133 episodes in 116 patients. Br J Haematol 1994 Apr; 86: 754–60
Nowoczyn V, Ritter J, Boos J, et al. Liposomal amphotericin B (ambisome) in neutropenic children with hematological malignancies and systemic fungal infections [abstract]. Med Pediatr Oncol 1992; 20(5): 376
Ringdén O, Tollemar J, Dahllöf G, et al. High cure rate of invasive fungal infections in immunocompromised children using ambisome. Transplant Proc 1994 Feb; 26: 175–7
Leenders ACAP, Daenen S, Jansen RLH, et al. Liposomal amphotericin B (AmBisome) compared with amphotericin B in the treatment of neutropenia-associated invasive fungal infections [abstract]. Trends in Invasive Fungal Infections 4: 1997 Nov 5; Barcelona
Tollemar J, Ringdén O, AmBisome(Rm) Users Group. Early pharmacokinetic and clinical results from a noncomparative multicentre trial of amphotericin B encapsulated in a small unilamellar liposome (Ambisome(Rm)). Drug Invest 1992; 4(3): 232–8
Ellis M, Spence D, Meunier F, et al. Randomised multicentre trial of 1 mg/kg (LD) versus 4 mg/kg (HD) liposomal amphotericin B (AmBisome) (LAB) in the treatment of invasive aspergillosis (IA): EORTC protocol 19923 [abstract no. LM39]. 36th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), American Society for Microbiology: 1996 Sep 15–19; New Orleans
Devecioglu Ö, Özgen Ü, Agaoglu L, et al. Clinical experience with amphotericin B (conventional & liposomal) in febrile neutropenic children [abstract]. Can J Infect Dis 1995 Jul; 6 Suppl. C 365C
Dornbusch HJ, Urban CE, Pinter H, et al. Treatment of invasive pulmonary aspergillosis in severely neutropenic children with malignant disorders using liposomal amphotericin B (AmBisome), granulocyte colony-stimulating factor, and surgery: report of five cases. Pediatr Hematol Oncol 1995 Nov–Dec; 12: 577–86
Pasic S, Flannagan L, Cant AJ. Liposomal amphotericin (AmBisome) is safe in bone marrow transplantation for primary immunodeficiency. Bone Marrow Transplant 1997; 19: 1229–32
Evdoridou J, Roilides E, Bibashi E, et al. Multifocal osteoarthritis due to Candida albicans in a neonate: serum level monitoring of liposomal amphotericin B and literature review. Infection 1997; 25(2): 44–8
Lazar JT, Ksionski GE. Efficacy of safety of AmBisome (liposomal amphotericin B) in primary episodes of cryptococcosis in patients with HIV infection [abstract no. W.B.2177]. 7th International Conference on AIDS: 1991 Jun 16–21; N
Mota-Miranda A, Gomes H, Marques R, et al. Liposomal amphotericin B in the treatment of opportunistic fungal infections. Rev Esp Quimioter 1994 Jun; 7: 146–9
Coker RJ, Viviani M, Gazzard BG, et al. Treatment of cryptococcosis with liposomal amphotericin B (AmBisome) in 23 patients with AIDS. AIDS 1993 Jun; 7: 829–35
Codeluppi M, Mussini C, Borghi V, et al. Liposomal amphotericin B in therapy in patients with AIDS [abstract no. P34]. Glasgow AIDS Meeting 1992 Nov
Leenders ACAP, Reiss P, Portegies P, et al. Liposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS-associated cryptococcal meniingitis. AIDS 1997; 11: 1463–71
Leggiadro RJ, Kline MW, Hughes WT. Extrapulmonary cryptococcosis in children with acquired immunodeficiency syndrome. Pediatr Infect Dis J 1991; 10: 658–62
Manfredi R, Coronado OV, Mastroianni A, et al. Liposomal amphotericin B and recombinant human granulocyte-macrophage colony stimulating factor (rHuGM-CSF) in the treatment of paediatric AIDS-related cryptococcosis. Int J STD AIDS 1997; 8: 406–8
Fisher NC, Mutimer DJ. Targeted and empirical liposomal amphotericin B therapy in liver transplantation: a 2-year review [abstract no. 1537]. Hepatology 1996; 24 (4, Pt. 2) Program Suppl.: 511A
Ng TTC, Denning DW. Liposomal amphotericin B (AmBisome) therapy in invasive fungal infections: evaluation of United Kingdom compassionate use data. Arch Intern Med 1995 May 22; 155: 1093–8
Munckhof W, Jones R, Tosolini FA, et al. Cure of Rhizopus sinusitis in a liver transplant recipient with liposomal amphotericin B [letter]. Clin Infect Dis 1993 Jan; 16: 183
Cofrancesco E, Boschetti C, Viviani MA, et al. Efficacy of liposomal amphotericin-B (AmBisome) in the eradication of Fusarium infection in a leukaemic patient. Haematologica 1992 May–Jun; 77: 280–3
Ellis ME, Clink H, Younge D, et al. Successful combined surgical and medical treatment of fusarium infection after bone marrow transplantation. Scand J Infect Dis 1994; 26: 225–8
Harten P, Baron Y, Euler HH. Liposomal amphotericin B therapy in disseminated histoplasmosis [letter]. Arch Intern Med 1995 Jul 24; 155: 1556
Berlanga JJ, Querol S, Gallardo D, et al. Successful treatment of Curvularia sp infection in a patient with primarily resistant acute promyelocytic leukemia. Bone Marrow Transplant 1995 Oct; 16: 617–9
Davidson RN, di Martino L, Gradoni L, et al. Liposomal amphotericin B (AmBisome) in Mediterranean visceral leishmaniasis: a multi-centre trial. Q J Med 1994 Feb; 87: 75–81
Davidson RN, di Martino L, Gradoni L, et al. Short-course treatment of visceral leishmaniasis with liposomal amphotericin B (AmBisome). Clin Infect Dis 1996 Jun; 22: 938–43
di Martino L, Davidson RN, Giacchino R, et al. Treatment of visceral leishmaniasis in children with liposomal amphotericin B. J Pediatr 1997; 131: 271–7
Russo R, Nigro LC, Minniti S, et al. Visceral leishmaniasis in HIV infected patients: treatment with high dose liposomal amphotericin B (AmBisome). J Infect 1996 Mar; 32: 133–7
Seaman J, Boer C, Wilkinson R, et al. Liposomal amphotericin B (AmBisome) in the treatment of complicated kala-azar under field conditions. Clin Infect Dis 1995 Jul; 21: 188–93
Thakur CP, Pandey AK, Sinha GP, et al. Comparison of three treatment regimens with liposomal amphotericin B (AmBisome Rm) for visceral leishmaniasis in India: a randomized dose-finding study. Trans R Soc Trop Med Hyg 1996 May–Jun; 90: 319–22
Ringdén O, Andström E, Remberger M, et al. Safety of liposomal amphotericin B (AmBisome) in 187 transplant recipients treated with cyclosporin. Bone Marrow Transplant 1994; 14 Suppl. 5: S10–14
Perfect JR, Lindsay MH, Drew RH. Adverse drug reactions to systemic antifungals: prevention and management. Drug Saf 1992 Sep–Oct; 7: 323–63
Meunier F, Prentice HG, Ringdén O. Liposomal amphotericin B (AmBisome): safety data from a phase II/III clinical trial. J Antimicrob Chemother 1991 Oct; 28 Suppl. B: 83–91
Bates CM, Carey PB, Hind CRK. Anaphylaxis due to liposomal amphotericin (AmBisome) [letter]. Genitourin Med 1995 Dec; 71: 414
Laing RBS, Milne LJR, Leen LJR, et al. Anaphylactic reactions to liposomal amphotericin [letter]. Lancet 1994 Sep 3; 344: 682
Arning M, Heer-Sonderhoff AH, Wehmeier A, et al. Pulmonary toxicity during infusion of liposomal amphotericin B in two patients with acute leukemia. Eur J Clin Microbiol Infect Dis 1995 Jan; 14: 41–3
Ringdén O, Andström E, Remberger M, et al. Allergic reactions and other rare side-effects of liposomal amphotericin [letter]. Lancet 1994 Oct 22; 344: 1156–7
Torre I, López-Herce J, Vázquez P. Anaphylactic reaction to liposomal amphotericin B in children. Ann Pharmacother 1996 Sep; 30: 1036–7
Aguado JM, Hidalgo M, Moya I, et al. Ventricular arrhythmias with conventional and liposomal amphotericin [letter]. Lancet 1993 Nov 13; 342: 1239
Goodwin SD, Cleary JD, Walawander CA, et al. Pretreatment regimens for adverse events related to infusion of amphotericin B. Clin Infect Dis 1995 Apr; 20: 755–61
Hoeprich PD. Clinical use of amphotericin B and derivatives: lore, mystique, and fact. Clin Infect Dis 1992 Mar; 14 Suppl. 1: S114–9
NeXstar Pharmaceuticals Limited. AmBisome: summary of product characteristics. Cambridge, England. 1996 Feb 07
NeXstar Pharmaceuticals Inc. Ambisome(R) (liposomal amphotericin B.P.): product monograph. Boulder, Colorado, USA, 1995
Pereira da Silva L, Videira Amaral JM, Cordeiro Ferreira N. Which is the most appropriate dosage of liposomal Amphotericin-B (AmBisome) for the treatment of fungal infections in infants of very low birth weight? [letter; comment]. Pediatrics 1993 Jun; 91: 1217–8
Lackner H, Schwinger W, Urban C, et al. Liposomal amphotericin-B (AmBisome) for treatment of disseminated fungal infections in two infants of very low birth weight. Pediatrics 1992 Jun; 89 (Pt 2): 1259–61
Leibovitz E, Juster A, Amitay A, et al. Liposomal amphotericin B (AmBisome) in the treatment of disseminated fungal infections in very low birth weight (VLBW) infants [abstract no. 4112]. 20th International Congress of Chemotherapy: 1997 Jun 29–Jul 3; Sydney, 128
Drugs used in the treatment of infection. British National Formulary. London: British Medical Association and the Royal Pharmaceutical Society of Great Britain, 1997 Sep: 230–292
Tollemar J, Ringdén O. Lipid formulations of amphotericin B: less toxicity but at what economic cost? Drug Saf 1995 Oct; 13: 207–18
Persson U, Tennvall GR, Andersson S, et al. Cost-effectiveness analysis of treatment with liposomal amphotericin B versus conventional amphotericin B in organ or bone marrow transplant recipients with systemic mycoses. Pharmacoeconomics 1992 Dec; 2: 500–8
Boogaerts M, Tormans G, Maes E, et al. Cost-effectiveness analysis of Ambisome (AMB) vs amphotericin B (AMPHOB) in the empiric treatment of febrile neutropenia in adults and children [abstract no. 55-IV]. Blood 1996 Nov 15; 88 Suppl. 1: 501a
Stewart A, Powles R, Hewetson M, et al. Costs of antifungal prophylaxis after bone marrow transplantation: a model comparing oral fluconazole, liposomal amphotericin and oral polyenes as prophylaxis against oropharyngeal infections. Pharmacoeconomics 1995 Oct; 8: 350–61
Richardson MD. Systemic fungal infections [68572]. Care Critically Ill 1994; 10: 258–61
Denning DW. Epidemiology and pathogenesis of systemic fungal infections in the immunocompromised host. J Antimicrob Chemother 1991; 28 Suppl. B: 1–16
Herbrecht R. The changing epidemiology of fungal infections: are the lipid-based forms of amphotericin B an advance? Eur J Haematol 1996; 56 Suppl. 57: 12–7
Uzun O, Anaissie EJ. Problems and controversies in the management of hematogenous candidiasis. Clin Infect Dis 1996; 22 Suppl. 2: S95–S101
Swerdloff JN, Filler SG, Edwards Jr. JE. Severe candidal infections in neutropenic patients. Clin Infect Dis 1993; 17 Suppl. 2: S456–67
Beyer J, Schwartz S, Heinemann V, et al. Strategies in prevention of invasive pulmonary aspergillosis in immunosuppressed or neutropenic patients. Antimicrob Agents Chemother 1994 May; 38: 911–7
Mahgoub ES. Agents of mycetoma. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious disease. 4th ed. ed. Vol. 2. New York: Churchill Livingstone, 1995: 2327–40
Powderly WG. Recent advances in the management of cryptococcal meningitis in patients with AIDS. Clin Infect Dis 1996; 22 Suppl. 2: S119–23
Abu-Salah KM. Amphotericin B: an update. Br J Biomed Sci 1996 Jun; 53: 122–33
British Society for Antimicrobial Chemotherapy Working Party. Antifungal chemotherapy in patients with acquired immunodeficiency syndrome. Lancet 1992; 340: 648–51
Gallis HA. Amphotericin B: a commentary on its role as an antifungal agent and as a comparative agent in clinical trials. Clin Infect Dis 1996 May; 22 Suppl. 2: S145–7
Gøtzsche PC, Johansen HK. Meta-analysis of prophylactic or empirical antifungal treatment versus placebo or no treatment in patients with cancer complicated by neutropenia. BMJ 1997; 314: 1238–44
Pearson RD, de Queiroz Sousa A. Leishmania species: visceral (kala-azar), cutaneous, and mucosal leishmaniasis. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious disease. 4th ed. ed. Vol. 2. New York: Churchill Livingstone, 1995: 2428–42
Gradoni L, Bryceson A, Desjeux P. Treatment of Mediterranean visceral leishmaniasis. Bull World Health Organ 1995; 73(2): 191–7
Ashford RW, Desjeux P, deRaadt P. Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitai Today 1992; 8(3): 104–5
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: J.D. Berman, Walter Reed Army Institute of Research, Walter Reed Army Medical Center, Washington, D.C., USA; C. Carbon, Assistance Publique, CHU Bichat Claude Bernard, Paris, France; R.J. Hay, St John’s Institute of Dermatology, Guy’s Hospital, London, England; W. Krüger, Knochenmarktransplantation, Abteilung Onkologie/Hämatologie, Universitäts-Krankenhaus Eppendorf, Hamburg, Germany; O. Ringdén, Department of Immunology, Microbiology, Pathology and Infectious Diseases, Huddinge University Hospital, Karolinska Institutet, Huddinge, Sweden; J. Tollemar, Departments of Transplantation Surgery and Clinical Immunology, Huddinge University Hospital, Karolinska Institutet, Huddinge, Sweden; G.G. Zhanel, Faculties of Medicine and Pharmacy, University of Manitoba, Department of Medicine, Health Sciences Centre, Winnipeg, Manitoba, Canada.
Rights and permissions
About this article
Cite this article
Coukell, A.J., Brogden, R.N. Liposomal Amphotericin B. Drugs 55, 585–612 (1998). https://doi.org/10.2165/00003495-199855040-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199855040-00008