Abstract
Synopsis
Irinotecan (CPT-11) is a semisynthetic derivative of camptothecin. It and other camptothecin analogues/derivatives appear to exert their antitumour activity by binding to topoisomerase I. The active metabolite of irinotecan, 7-ethyl-10-hydroxycamptothecin (SN-38), has demonstrated potent growth inhibition of human colorectal cancer cells in vitro, with superior activity to fluorouracil.
In phase II clinical studies in patients with advanced colorectal cancer, objective response rates after irinotecan therapy ranged between 20.5 and 32%. These studies used a range of irinotecan regimens including 350 mg/m2 once every 3 weeks (Europe), 125 to 150 mg/m2 once a week for 4 weeks followed by a 2-week drug-free interval (US) and 100 mg/m2 /week or 150 mg/m2 every 2 weeks (Japan). The median duration of response ranged between 5.6 and 10.6 months. Disease stabilisation occurred in 30 to 71.2% of patients. Objective response rates to irinotecan therapy in patients who had received no prior chemotherapy were similar to those in patients pretreated with fluorouracil. Importantly, irinotecan also induced responses in some patients with tumours refractory to fluorouracil.
Severe (grade 3 or 4) neutropenia and diarrhoea, which occurred in up to 40% of patients receiving irinotecan therapy in phase II studies, require careful monitoring and appropriate management.
Thus, irinotecan is a valuable agent for the second-line treatment of patients with advanced colorectal cancer who fail to respond to or relapse after fluorouracil therapy.
Pharmacodynamic Properties
The antitumour agent irinotecan is a semisynthetic derivative of camptothecin. Irinotecan has shown growth inhibitory activity in vitro against human colon tumour cells and freshly explanted colorectal tumours; however, its metabolite 7-ethyl-10-hydroxycamptothecin (SN-38) had considerably greater activity. SN-38 had similar potency to camptothecin and was a more potent inhibitor of human colon cell line growth than fluorouracil, mitomycin or cisplatin in vitro. Irinotecan produced 70 to 85% inhibition of growth of human colon tumour xenografts implanted in nude mice.
No synergistic effect on antitumour activity was observed when irinotecan was administered either before or after fluorouracil or etoposide in immunedeprived mice bearing human tumour xenografts.
Tumours overexpressing the multidrug-resistance gene mdr1 generally remain sensitive to the camptothecin class of drugs.
Mechanisms proposed to account for resistance to irinotecan include reduced topoisomerase I levels in resistant compared with parent tumour cells, reduced topoisomerase I activity and reduced hydrolysis of irinotecan to its metabolite SN-38.
Pharmacokinetic Properties
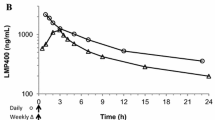
Irinotecan is metabolised by the carboxylesterase enzyme system to its active metabolite SN-38. Peak plasma concentrations (Cmax) of irinotecan are reached by the end of intravenous infusion, whereas those of SN-38 occur about 0.5 to 2 hours after the infusion period. Cmax values for irinotecan increased in a dose-dependent manner, ranging between 1.3 and 2.3 mg/L following administration of a 100 mg/m2 dose of irinotecan. There was significant interpatient variation in the area under the plasma concentration-time curve (AUC). AUC values for SN-38 generally correlated with those of irinotecan.
Irinotecan had a large mean volume of distribution, ranging between 104 and 211 L/m2, after intravenous infusion of 100 to 350 mg/m2.
Both irinotecan and SN-38 are subject to pH-dependent hydrolysis of the active closed lactone ring structure to an inactive open ring carboxylate. The AUC ratio of active closed ring form versus total varied from 33.9 to 44% and from 44.7 to 64%, respectively, for irinotecan and SN-38.
Plasma clearance of irinotecan was between 11 and 26 L/m2 /h and the mean residence time was generally between 9 and 12 hours, independent of dose.
Elimination of irinotecan was triphasic, the first elimination phase lasting 1.2 to 12 minutes and the second phase 1.2 to 2.5 hours. The terminal elimination half-life (t1/4) was between 5.5 and 12.5 hours. SN-38 generally had a longer t1/4: 7.7 to 17 hours. 10 to 26% of an administered dose of irinotecan and 0.18 to 0.26% of SN-38 were eliminated in the urine over a 24-hour period.
Coadministration of irinotecan and fluorouracil slightly decreased the metabolism of irinotecan to its active metabolite SN-38 early in 1 study; However, this reduction was not evident later in the trial and is unlikely to be clinically important.
Clinical Efficacy
Phase I studies investigated the maximum tolerable dose (MTD) of irinotecan and the optimal dosage regimen in patients with a range of malignancies. Irinotecan 350 mg/m2 once every 3 weeks was associated with a lower incidence of limiting toxicity (diarrhoea and neutropenia — see tolerability summary) than other dosage regimens investigated in Europe. In the US, dosages of 240 mg/m2 once every 3 weeks or 150 mg/m2 every week for 4 weeks followed by a 2-week drug-free interval were considered optimal; in Japan, MTDs were 30 mg/m2 /day by continuous intravenous infusion for 5 days or 200 mg/m administered once every 3 to 4 weeks.
Phase II clinical trials were conducted in patients with advanced colorectal cancer who were either chemotherapy-naive or had received previous therapy with fluorouracil. Irinotecan 350 mg/m once every 3 weeks induced an objective response in 20.5% of 156 evaluable patients in an initial European study; the median duration of response was 9.1 months and the 1-year survival rate was 43.1%. Objective response rates in 3 subsequent trials ranged between 6.5 and 14%.
In US studies, in which irinotecan 100 to 150 mg/m2 was administered once weekly for 4 weeks followed by a 2-week drug-free interval, objective response rates ranged between 8.6 and 32% and the median duration of response was 5.6 to 8.1 months. Disease stabilisation occurred in 30 to 71.2% of patients.
In a Japanese study, irinotecan 100 mg/m2 /week or 150 mg/m2 once every 2 weeks induced responses in 27% of patients with a median duration of 10.6 months. Disease stabilisation was observed in 30% of patients.
Tolerability
Digestive system events such as diarrhoea, nausea and vomiting were common drug-related adverse events in the 304 patients treated with irinotecan in US phase II trials. Other common adverse events included asthenia, abdominal pain, leuco-penia and neutropenia. At least 1 of these adverse events occurred in >50% of patients; <31% of patients had adverse events of grade 3 or 4 severity. The most common grade 3 or 4 adverse event was delayed diarrhoea, which was reported in 93 of 304 (30.6%) patients. Grade 3 or 4 nausea occurred in 51 (16.8%) patients.
Irinotecan caused grade 3 or 4 neutropenia in 80 (26.3%) patients but clinical complications arising from myelosuppression were relatively infrequent. Nine patients developed neutropenic fever (concurrent grade 4 neutropenia and ≥ grade 2 fever). Nine patients who received irinotecan during US phase II trials discontinued therapy because of adverse events (diarrhoea and/or neutropenia/leucopenia) considered by the investigator(s) to be drug-related. There was 1 death possibly attributable to a drug-related adverse event (neutropenia).
The most common adverse events in European phase II studies were delayed diarrhoea (grade 3 or 4 in 38.5% of patients), cholinergic syndrome (including early diarrhoea) [grade 3 or 4 in 10.3% of patients], nausea and/or vomiting (grade 3 or 4 in 18.9% of patients), neutropenia (grade 3 or 4 in 39.6% of patients), asthenia and alopecia.
There were 66 episodes of fever and/or infection associated with grade 3 or 4 neutropenia; 2 patients died with sepsis associated with grade 3 or 4 neutropenia.
Most patients experienced alopecia and ≈50% experienced complete hair loss.
Dosage and Administration
The recommended dosage of irinotecan for the treatment of patients with advanced colorectal cancer in Europe is 350 mg/m2 once every 3 weeks administered as a ≥30-minute intravenous infusion. The dosage should be reduced in patients who experience severe neutropenia or develop infections associated with neutropenia. Treatment should be delayed until full recovery in cases of severe neutropenia or severe gastrointestinal adverse events.
The recommended initial dose of irinotecan in the US is 125 mg/m2 administered as a 90-minute intravenous infusion. The recommended treatment regimen is 1 weekly dose for 4 weeks followed by a 2-week drug-free interval. This 6-week regimen may be repeated; subsequent doses should be adjusted upwards (to as high as 150 mg/m2) or downwards (to as low as 50 mg/m2) in 25 to 50 mg/m2 steps depending on tolerance of treatment. Treatment can be continued indefinitely provided patients do not develop intolerable adverse events.
Contraindications include chronic inflammatory bowel disease, hypersensitivity to irinotecan or one of its excipients, pregnancy and lactation.
Similar content being viewed by others
References
Jaxel C, Kohn KW, Wani MC, et al. Structure-activity study of the actions of camptothecin derivatives on mammalian topoisomerase I: evidence for a specific receptor site and a relation to antitumor activity. Cancer Res 1989; 49: 1465–9
Sinha BK. Topoisomerase inhibitors: a review of their therapeutic potential in cancer. Drugs 1995 Jan; 49: 11–9
Gupta M, Fujimori A, Pommier Y. Eukaryotic DNA topoisomerases I. Biochim Biophys Acta Gene Struct Expr 1995; 1262: 1–14
Pommier Y. Eukaryotic DNA topoisomerase I: Genome gatekeeper and its intruders, camptothecins. Semin Oncol 1996; 23(1) Suppl. 3: 3–10
Giovanella PC, Stehlin JS, Wall ME, et al. DNA topoisomerase I-targeted chemotherapy of human colon cancer in xeno-grafts. Science 1989; 246: 1046–8
Wall ME, Wani MC, Cook CE, et al. Plant antitumour agents. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J Am Chem Soc 1966; 88: 3888–90
Shimada Y, Rothenburg M, Hilsenbeck SG, et al. Activity of CPT-11 (irinotecan hydrochloride), a topoisomerase I inhibitor, against human tumor colony-forming units. Anticancer Drugs 1994 Apr; 5: 202–6
Tanizawa A, Fujimori A, Fujimori Y, et al. Comparison of topoisomerase I inhibition, DNA damage, and cytotoxicity of camptothecin derivatives presently in clinical trials. J Natl Cancer Inst 1994 Jun 1; 86: 836–42
Funakoshi S, Aiba K, Shibata H, et al. Enhanced antitumor activity of SN-38, an active metabolite of CPT-11, and 5-fluorouracil combination for human colorectal cancer cell lines [abstract]. Proc Am Soc Clin Oncol 1993 Mar; 12: 193
Aiba K, Funakoshi S, Mizunuma N, et al. Antitumor effect of SN-38, active form of CPT-11, on human colorectal cancer cell line [in Japanese]. Gan to Kagaku Ryoho 1994 Aug; 21: 1601–6
Hirano A, Sano M, Funakoshi S, et al. An investigation of optimal dose schedules of CPT-11, a camptothecin derivative in human carcinoma cell lines [abstract]. Proc Am Assoc Can Res 1993; 34: 420
Kawato Y, Furuta T, Aonuma M, et al. Antitumor activity of a camptothecin derivative, CPT-11, against human tumor xeno-grafts in nude mice. Cancer Chemother Pharmacol 1991 Jul; 28: 192–8
Houghton PJ, Cheshire PJ, Hallman JC, et al. Therapeutic efficacy of the topoisomerase I inhibitor 7-ethyl-10-(4-[1-piperidino] -1-piperidino)-carbonyloxy-camptothecin against human tumor xenografts: lack of cross-resistance in vivo in tumors with acquired resistance to the topoisomerase I inhibitor 9-dimethylaminomethyl- 10-hydroxycamptothecin. Cancer Res 1993 Jun 15; 53: 2823–9
Houghton PJ, Cheshire PJ, Hallman II JD, et al. Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemother Pharmacol 1995 Sep; 36: 393–403
Tsunoda A, Shibusawa M, Takada M, et al. A model for sensitivity determination of anticancer agents against chemical-induced colon cancer in rats [in Japanese]. Gan to Kagaku Ryoho 1995 Sep; 22: 1363–7
Guichard S, Caliaro MJ, Houin G, et al. Effet synergique de l’exposition séquentielle de CPT-11 et de 5-FU sur une lignée d’adénocarcinome colique humain HT-29 [abstract]. Bull Cancer 1996; 83: 436–7
Houghton JA, Cheshire PJ, Hallman II JD, et al. Evaluation of irinotecan in combination with 5-fluorouracil or etoposide in xenograft models of colon adenocarcinoma and rhabdomyosarcoma. Clin Cancer Res 1996 Jan; 2: 107–18
Kim R, Hirabayashi N, Nishiyama M, et al. Experimental studies on biochemical modulation targeting topoisomerase I and II in human tumor xenografts in nude mice. Int J Cancer 1992 Mar 12; 50: 760–6
Grossin F, Barbault H, Benhammouda A, et al. Association concomitante de CPT 11 (C)/5-flouorouracile (F): étude pharmacocinetique de phase I [abstract]. Bull Cancer 1996; 83: 475–6
Chen AY, Yu C, Potmesil M, et al. Camptothecin overcomes MDR1-mediated resistance in human KB carcinoma cells. Cancer Res 1991; 51: 6039–44
Tsuruo T, Matsuzaki T, Matsushita M, et al. Antitumor effect of CPT-11, a new derivative of camptothecin, against pleiotropic drug-resistant tumors in vitro and in vivo. Cancer Chemother Pharmacol 1988; 21: 71–4
Mattern MR, Hofmann GA, Polsky RM, et al. In vitro and in vivo effects of clinically important camptothecin analogues on multidrug-resistant cells. Oncol Res 1993; 5(12): 467–74
Nagai S, Yamauchi M, Andoh T, et al. Establishment and characterization of human gastric and colonic xenograft lines resistant to CPT-11 (a new derivative of camptothecin). J Surg Oncol 1995 Jun; 59: 116–24
Sugimoto Y, Tsukahara S, Ohhara T, et al. Decreased expression of DNA topoisomerase I in camptothecin-resistant tumor cell lines as determined by a monoclonal antibody. Cancer Res 1990 Nov 1; 50: 6925–30
Saijo N, Nishio K, Kubota N, et al. 7-Ethyl-10-[4-(1-piperidino)-1-piperidino] carbonyloxy camptothecin: mechanism of resistance and clinical trials. Cancer Chemother Pharmacol 1994 Aug; 34 Suppl.: 112–7
Abigerges D, Chabot GG, Armand J-P, et al. Phase I and pharmacologic studies of the camptothecin analog irinotecan administered every 3 weeks in cancer patients. J Clin Oncol 1995 Jan; 13: 210–21
Catimel G, Chabot GG, Guastalla JP, et al. Phase I and pharmacokinetic study of irinotecan (CPT-11) administered daily for three consecutive days every three weeks in patients with advanced solid tumors. Ann Oncol 1995 Feb; 6: 133–40
Rothenberg ML, Kuhn JG, Burris III HA, et al. Phase I and pharmacokinetic trial of weekly CPT-11. J Clin Oncol 1993 Nov; 11: 2194–204
Rowinsky EK, Grochow LB, Ettinger DS, et al. Phase I and pharmacological study of the novel topoisomerase I inhibitor 7-ethyL-10-[4-(1-piperidino)-1-piperidino]carbonyloxycam ptothecin (CPT-11) administered as a ninety-minute infusion every 3 weeks. Cancer Res 1994 Jan 15; 54: 427–36
Canal P, Gay C, Dezeuze A, et al. Pharmacokinetics and pharmacodynamics of irinotecan hydrochloride (CPT-11) during a phase II clinical trial in colorectal cancer. J Clin Oncol. In press
de Forni M, Bugat R, Chabot GG, et al. Phase I and pharmacokinetic study of the camptothecin derivative irinotecan, administered on a weekly schedule in cancer patients. Cancer Res 1994 Aug 15; 54: 4347–54
SaLtz LB, Kanowitz J, KeMeny NE, et al. A phase I clinical and pharmacokinetic study of irinotecan HCL (Irinotecan, CPT-11), 5-fluorouracil and leucovorin in patients with advanced solid tumours. J Clin Oncol. In press
Rivory LP, Chatelut E, Canal P, et al. Kinetics of the in vivo interconversion of the carboxylate and lactone forms of irinotecan (CPT-11) and of its metabolite SN-38 in patients. Cancer Res 1994 Dec 15; 54: 6330–3
Sasaki Y, Ohtsu A, Shimada Y, et al. Simultaneous administration of CPT-11 and fluorouracil: alteration of the pharmacokinetics of CPT-11 and SN-38 in patients with advanced colorectal cancer. J Natl Cancer Inst 1994 Jul 20; 86: 1096–8
Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981; 47: 207–14
Creekmore SP, Urba WJ, Longo DL. Principles of the clinical evaluation of biologic agents. In: DeVita VT, Hellman S, Rosenberg SA, editors. Biologic therapy of cancer. Philadelphia: Lippincott, 1991: 67–86
Ohe Y, Sasaki Y, Shinkai T, et al. Phase I study and pharmacokinetics of CPT-11 with 5-day continuous infusion. J Natl Cancer Inst 1992 Jun 17; 84: 972–4
Taguchi T, Wakui A, Hasegawa K, et al. Phase I clinical study of CPT-11 [in Japanese]. Gan to Kagaku Ryoho 1990 Jan; 17: 115–20
Bleiberg H. Open-label confirmatory Multicentre phase II study of irinotecan-hydrochloride (CPT-11) in patients with 5-FU-resistant colorectal cancer (Study number: RP 64174A-V-222). Rhone-Poulenc Rorer Data on fIle 1996 Feb 29
Herait P, Tavakoli F. Integrated summary efficacy (irinotecan hydrochloride trihydrate, RP 64174 A). March 1996 update. Rhone-Poulenc Rorer Data on fIle 1996 Feb 28
Van Cutse ME, Cunningham D, Ten Bokkel Huinink W, et al. Irinotecan (CPT-11) multicenter phase II study in colorectal cancer patients with documented progressive disease on prior 5FU: preliMinlary results [abstract no. 562]. Proc Am Soc Clin Oncol 1996 Mar; 15: 230
Rougier P, Culine S, Bugat R, et al. A phase II study of CPT-11 (irinotecan) in the treament of advanced colorectal cancer in chemothereapy-naive patients and patients pretreated with 5-FU-based chemotherapy. J Clin Oncol In press
Conti JA, Kemeny NE, Saltz LB, et al. Irinotecan (CPT-11) is an active agent in untreated patients with metastatic colorectal cancer. J Clin Oncol 1996 Mar; 14(3): 709–15
Deitz AJ, Von Hoff DD, Ragual RH, et al. A multicenter, phase II study of irinotecan hydrochloride (CPT-11) in metastatic colorectal carcinoma refractory to previous 5-fluoruracil (5-FU)-based chemotherapy (protocol M/6475/0006). Upjohn Technical Report 1995 Dec 1; 7216-95-008
Dietz AJ, Von Hoff DD, Elfring GL, et al. A multicenter openlabel, phase II study of irinotecan hydrochloride (CPT-11) in patients with 5-fluorouracil (5-FU)-refractory colorectal cancer (protocol M/6475/0006). Upjohn Technical Report 1995 Dec 1; 7216-95-010
Pitot HC, Wender D, O’Connell MJ, et al. A phase II trial of CPT-11 (irinotecan) in patients with metastatic colorectal carcinoma: a North Central Cancer Treatment Group study [abstract]. Proc Am Soc Clin Oncol 1994 Mar; 13: 197
Rothenberg ML, Eckardt JR, Kuhn JG, et al. Phase II trial of irinotecan in patients with progressive or rapidly recurrent colorectal cancer. J Clin Oncol 1996 Apr; 14(4): 1128–35
Dietz AJ, Von Hoff DD, Elfing GL, et al. A phase II study of irinotecan hydrochloride (CPT-11) in metastatic colorectal carcinoma refractory to previous 5-fluorouracil (5-FU)-based chemotherapy (protocol M/6475/0001). Upjohn Technical Report 1995 Dec 1; 7216-95-007
Shimada Y, Yoshino M, Wakui A, et al. Phase II study of CPT-11, a new camptothecin derivative, in metastatic colorectal cancer. J Clin Oncol 1993 May; 11: 909–13
Irinotecan hydrochloride: Integrated summary of safety, March 1996 update. Data on file Rhone-Poulenc Rorer, Antony, France. 1996 Mar
Von Hoff DD. Expert report on the clinical documentation. Irinotecan hydrochloride injection for the treatment of previously treated metastatic carcinoma of the colon or rectum. Data on file Pharmacia & Upjohn Inc. Kalamazoo, Michigan, USA. 1996 May 3
Rhone Poulenc Rorer. Irinotecan prescribing information, Antony, France. 1996
Pharmacia & Upjohn, Inc. Irinotecan prescribing information, Kalamazoo, Michigan, USA. 1995
Schmoll H-J. Colorectal carcinoma: current problems and future perspectives. Ann Oncol 1994; 5 Suppl. 3: S115–121
Connors TA, Duncan R, Knox RJ. The chemotherapy of colon cancer. Eur J Cancer A 1995 Jul–Aug; 31A: 1373–8
Innovative drug for colorectal cancer now available in France. Anticancer Drugs 1996 Jan; 7: 131
Moertel CG. Chemotherapy for colorectal cancer. N Engl J Med 1994; 330: 1136–42
van Triest B, van Groeningen CJ, Pinedo HM. Current chemotherapeutic possibilities in the treatment of colorectal cancer. Eur J Cancer 1995; 31A: 1193–7
Advanced colorectal cancer meta-analysis project: modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer. Evidence in terms of response rate. J Clin Oncol 1992; 10: 896–903
Buroker TR, O’connell MJ, Wieand HS, et al. Randomised comparison of two schedules of fluorouracil and leucovorin in the treatment of advanced colorectal cancer. J Clin Oncol 1994; 12(1): 14–20
Kohne CH, Wilke H, Hecker H, et al. Interferon-alpha does not improve the antineoplastic efficacy of high-dose infusional 5-flourouracil plus folinic acid in advanced colorectal cancer. First results of a randomized multicenter study by the association of medical oncology of the German cancer society (AIO). Ann Oncol 1995 May; 6: 461–6
Ahlgren JD, Trocki O, Gullo JJ, et al. Protracted infusion of 5-FU with weekly low-dose cisplatin as second-line therapy in patients with metastatic colorectal cancer who have failed 5-FU monotherapy. Cancer Invest 1991; 9: 27–33
Bertrand M, Doroshow JH, Multhauf P, et al. High-dose continuous infusion folinic acid and bolus 5-fluorouracil in patients with advanced colorectal cancer: a phase II study. J Clin Oncol 1986 Jul; 4(7): 1058–61
DeLap R, Bodurian E, Nazzaro D, et al. PALA plus 5-FU in colorectal cancer; pilot clinical study of a convenient outpatient treatment regimen [abstract]. Proc Am Assoc Can Res 1993 Mar; 34: 285
Izzo J, Cvitkovic E, Zarba J, et al. Low dose 5FU continuous infusion (FuCI) in advanced colo rectal cancer (ACC): clinical evidence for reversal of aquired/intrinsic resistance to 5 FU or 5 FU folinic (FUFo) [abstract no. 76]. Ann Oncol 1992; 3 Suppl. 5: 20
Leong L, Doroshow J, Akman S, et al. Phase II trial of 5-FU and high dose folinic acid (HDFA) with cis-platin (CDDP) and dipyridamole (DP) in advanced colorectal cancer [abstract no. *383]. Proc Am Soc Clin Oncol 1989 Mar; 8: 99
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: J.P. Armand, Département de Médecine, Institut Gustave-Roussy, Villejuif, France; G. Catimel, Center Regional Léon Bérard, Lyon, France; P.J. Houghton, Department of Molecular Pharmacology, St Jude Children’s Research Hospital, Memphis, Tennessee, USA; R. Knox, CRC Centre for Cancer Therapeutics at the Institute of Cancer Research, London, England; Y. Pommier, National Institutes of Health, Bethesda, Maryland, USA; L.P. Rivory, Department of Medicine, Princess Alexandra Hospital, University of Queensland, Brisbane, Queensland, Australia; N. Saijo, National Cancer Center Research Institute, Tokyo, Japan; L.B. Saltz, Division of Solid Tumor Oncology, Memorial Sloan-Kettering Cancer Center, New York, New York, USA; H.J. Schmoll, Klinik und Poliklinik für Innere Medizin, Medizinische Fakultät, Martin-Luther-Universität Halle-Wittenberg, Halle, Germany; Y. Shimada, National Cancer Center Hospital, Tokyo, Japan; B.K. Sinha, Biochemical and Molecular Pharmacology Section, Clinical Pharmacology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA.
Rights and permissions
About this article
Cite this article
Wiseman, L.R., Markham, A. Irinotecan. Drugs 52, 606–623 (1996). https://doi.org/10.2165/00003495-199652040-00013
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199652040-00013