Abstract
Synopsis
Pentostatin, a potent inhibitor of adenosine deaminase, is an antineoplastic agent which has been studied in the treatment of a variety of lymphoproliferative disorders. It is particularly effective in the treatment of hairy cell leukaemia, achieving complete remissions in 33 to 92% of patients, and has useful activity in treating B cell chronic lymphocytic leukaemia, prolymphocytic leukaemia, adult T cell leukaemia/lymphoma and cutaneous T cell lymphoma refractory to conventional chemotherapy. Initial results suggest that in the treatment of hairy cell leukaemia pentostatin achieves a more rapid response and higher frequency of complete remission with longer duration than interferon-α2a, although it is still not known if some patients experiencing complete remission have been cured. The drug has yet to be directly compared with other promising purine analogues such as cladribine and fludarabine, and results of such comparisons are required before the ultimate role of pentostatin in the treatment of hairy cell leukaemia can be clearly established. However, pentostatin does produce a substantial response in a difficult therapeutic area and should be considered for initial treatment of hairy cell leukaemia.
Pharmacodynamic Properties
Pentostatin is a potent inhibitor of adenosine deaminase (ADA), the purine salvage enzyme involved in the irreversible deamination of adenosine and deoxyadenosine. ADA is detected in virtually all mammalian tissues with greatest activity in those of the lymphoid system, and is increased in the blast cells of patients with acute lymphocytic or myelogenous leukaemia. In vitro, pentostatin binds to ADA with high affinity and inhibits activity of the enzyme in animal tissue and human chronic myelogenous cells. Intravenous administration of pentostatin 0.1 to 1 mg/ kg to patients with acute lymphoblastic leukaemia caused a dose-related inhibition of lymphoblast ADA activity, this activity became undetectable on day 2 after a single pentostatin dose and remained below half of the pretreatment values for 2 weeks, although plasma ADA activity returned to normal within 24 hours. Cellular deoxyadenosine triphosphate (dATP) levels increased concomitantly with inhibition of ADA following pentostatin administration, there being a strong correlation between dATP accumulation and lymphoblast lysis. However, therapeutic responses occurred in only some patients in whom ADA was inhibited.
In vitro, pentostatin alone exhibited minimal activity against animal and human leukaemic cell lines, requiring the presence of a purine nucleoside (deoxyadenosine, dAdo) to inhibit cell growth and deoxyribonucleic acid (DNA) synthesis. Human T cell lines were more sensitive to the toxic effect of pentostatin plus dAdo than B cell lines. The increased sensitivity of the T cell lines was associated with accumulation of dATP and reduction of ATP, there being a correlation between LD50 (concentration of dAdo that caused a 50% inhibition of [3H]thymidine incorporation in the presence of pentostatin 20 μmol/L) and the ratio of dATP to ATP.
Pentostatin combined with interferon (IFN)-α2 but not with IFN-γ, had synergistic activity against some human leukaemic cell lines.
Immunosuppression is produced by pentostatin in animals and in humans. In patients with hairy cell leukaemia (HCL) administration of pentostatin 2 to 4 mg/m2 at intervals of 2 to 6 weeks was preferentially lymphotoxic, with T and B cells both reduced. Natural killer cells were reduced to a lesser extent than T-helper and suppressor cells. Mean counts of CD4+ T cells were reduced to <200/μl and remained reduced for many months after cessation of pentostatin. Lymphocyte proliferative response to stimulation by mitogens and alloantigens decreased concomitantly with the reduction in lymphocytes and their subpopulations, but in one study in patients with HCL treated with pentostatin, production of interleukin-2 and tumour necrosis factor remained normal, as did IFN production and T cell proliferative response induced by all but one mitogen tested. Despite prolonged suppression of CD4+ T cells there were no severe opportunistic infections nor increased incidence of secondary malignancies in patients with HCL that responded to treatment with pentostatin.
The mechanism of action of pentostatin is not completely clear, but major proposed mechanisms relate to accumulation of dATP, and the consequent inhibition of ribonucleotide reductase and depletion of other deoxynucleotides, resulting in inhibition of DNA synthesis. While this mechanism may be appropriate for rapidly proliferating cells, it does not explain the toxicity of pentostatin to slowly proliferating cells. Other sites of action have been proposed subsequent to the finding of single-strand breaks in the DNA of cells treated with pentostatin plus dAdo. The importance of accumulated dATP in mediating cytotoxicity has also been questioned, and a decrease in ATP subsequent to depletion of nicotinamide adenine dinucleotide and increased formation of 2′,5′-oligoadenylate have been suggested as alternative mechanisms. However, none of these changes in nucleotide or enzyme levels is predictive of clinical response, whereas a correlation of clinical response with decreased S-adenosylhomocysteine levels has been suggested. Other suggestions relate to the requirement for phosphorylation of dAdo to inhibit repair of DNA strand breaks and the possible role of natural killer activity.
Pharmacokinetic Properties
The pharmacokinetic properties of pentostatin have been studied in small numbers of patients with lymphoproliferative disorders treated with the drug. Mean plasma concentrations of 2 to 6 μmol/L were observed 1 hour after intravenous administration of pentostatin 0.25 to 1 mg/kg, and were proportional to dose.
Pentostatin penetrates the blood-brain barrier, with concentrations in cerebrospinal fluid reaching 10 to 12% of those in plasma 2 to 4 hours after intravenous administration. Mean apparent volumes of distribution during the terminal phase and at steady-state were 42.4 and 36.9L, respectively.
After intravenous administration of pentostatin 5 to 30 mg/m2 to patients, 50 to 82% of the dose was recovered in the urine on day 1, and in another study, 96% of the administered dose was recovered as either unchanged drug or compounds inhibiting ADA activity over a period of 24 hours.
Plasma elimination half lives between 3 and 9.4 hours were reported when a sampling period of 24 hours was used.
Therapeutic Efficacy
The therapeutic efficacy of pentostatin has been studied in mostly phase II trials in generally small groups of patients with a variety of lymphoproliferative malignancies.
In the treatment of HCL in patients with or without splenectomy, and in those previously or not treated with IFN-α, pentostatin achieved a complete response (CR) in 33 to 92% of patients. Usual dosage was 4 mg/m2 every 2 weeks, but optimum dosage has yet to be determined. In some studies, the proportion of patients with CR varied according to the nature of the previous response to IFN, with a greater CR or partial response (75%) in those who were intolerant of IFN-α, or whose disease progressed after an initial response to IFN, compared with those who failed to respond to IFN (35%). The criteria used to define response or CR also influenced the proportion of patients responding. Response to pentostatin was similar irrespective of whether or not patients had previously undergone splenectomy.
In nearly all patients, improvement in peripheral blood counts occurred after about 2 cycles of treatment. The median time to best response was about 4 months. The duration of follow-up and consequently the reported duration of remission varied between studies. In the longest follow-up to date, 56% of patients had relapsed, but all patients with CR remained alive and well after a mean period of about 6 years. Retreatment was not required, reappearance of hairy cells was confined to the bone marrow, and peripheral blood counts remained normal.
To date, there are no data from appropriately designed trials on the effect of treatment with pentostatin on survival in patients with HCL who achieve CR.
Pentostatin and IFN-α are clinically non-cross-resistant but it is unclear, from preliminary results, whether coadministration of these agents improves the response rate or significantly decreases the incidence of serious infection relative to low dose pentostatin alone.
The efficacy of pentostatin in the treatment of chronic lymphocytic leukaemia of B cell origin (B-CLL) has been studied in small numbers of patients, most of whom were previously treated with one of more regimens of conventional therapy, had stage III to IV disease (Rai system), and were in performance class 0 to 2. Response to pentostatin 4 mg/m2 weekly or every 2 weeks was moderate, with CR achieved in 0 to 4%, PR in 15 to 27%, and clinical improvement in a further 15 to 30% of patients. Response appeared to be related to the extent of previous treatment, being somewhat higher in patients without prior treatment compared with those treated at least once previously. Some patients not regarded as responders had palliative clinical benefit evidenced by decreased blood transfusion requirements.
Pentostatin 4 mg/m2 weekly for the first 3 or 4 weeks, then every 2 weeks for 6 weeks or until optimal response, has achieved promising results in small numbers of patients with prolymphocytic leukaemia. Partial response was obtained in 58%, with complete response in up to 10% of patients. The median survival was 16 months in patients with CR or PR, and 10 months in nonresponders with this difficult-to-treat, rare disease.
Treatment regimens used in CLL are generally not effective in adult T cell leukaemia/lymphoma, and pentostatin has been studied in small groups of patients with this disease. Studies conducted in the Caribbean and Japan reported response in 20 to 32% of patients generally treated with pentostatin 4 or 5 mg/m2 for 2 or 3 consecutive days, followed by administration every 1 or 2 weeks.
In children with relapsed acute lymphoblastic leukaemia (ALL), continuous infusion of pentostatin 20 to 30 mg/m2 daily for 1 to 3 days achieved CR in about 10% and oncolytic response in a further 42% of patients. When used in conjunction with vidarabine, pentostatin appeared ineffective in prolonging the duration of a second CR achieved with conventional chemotherapy in children with relapsed ALL.
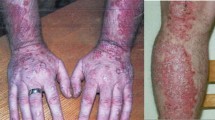
Pentostatin has been used to treat a variety of relapsed or refractory non-Hodgkin’s lymphomas including mycosis fungoides and Sézary syndrome (collectively referred to as cutaneous T cell lymphoma; CTCL). In small numbers of patients with CTCL treatment with pentostatin has achieved CR or PR in 17 to 41% of patients, mostly those with previously treated disease. In other non-Hodgkin’s lymphomas, pentostatin treatment has achieved CR in 0 to 20% and PR in 10 to 30% of patients.
Tolerability
In early clinical trials, considerable toxicity involving the renal, central nervous and immune systems was associated with high dosages of pentostatin. However, toxicity has been considerably reduced by lower dosages used intermittently.
Myelosuppression occurs frequently during administration of pentostatin, but in patients with leukaemia appeared to be a function of the underlying disease rather than of treatment, and was usually transient, with blood cell counts returning towards normal as the leukaemia responded to treatment. The principal dose-limiting toxicity was generally neutropenia, which was severe in about 16 to 25% of patients and life-threatening in 25 to 70% when graded according to Eastern Cooperative Oncology Group criteria. However, in patients without leukaemia and normal blood elements, post-treatment myelosuppression was less marked.
The incidence of infection resulting from both bacterial pathogens and opportunistic organisms in patients with neoplastic diseases treated with pentostatin has varied from about 8 to 58%. Opportunistic infections occurred frequently in patients with CLL, but were less common, or absent, in patients with HCL. Usually, infections were fatal in about 3 to 6% of patients with HCL treated with pentostatin.
Nausea and/or vomiting, ranging in severity from mild to life-threatening have occurred in 36 to 87% of patients, while skin rash, frequently of mild to moderate severity, has been reported in 6 to 67% of patients. At currently used dosages, central nervous system effects are usually confined to fatigue, headaches, malaise and depression, and renal and hepatic dysfunction are usually mild to moderate and often transient.
Dosage and Administration
The recommended adult dosage of pentostatin for the treatment of hairy cell leukaemia, including that refractory to IFN-α, is 4 mg/m2 administered intravenously every 2 weeks. The optimum duration of treatment has yet to be determined, but in the absence of major toxicity treatment should continue until a complete response has occurred. In some studies an additional 2 pentostatin doses were administered after a complete response was achieved.
Similar content being viewed by others
References
Agarwal RP. Pharmacology of 2′-deoxycoformycin and vidarabine. Cancer Treatment Symposia 2 17–22, 1984
Agarwal RP, Spector T, Parks RE. Tight binding inhibitors — IV. Inhibition of adenosine deaminases by various inhibitors. Biochemical Pharmacology 26: 359–367, 1977
Anonymous. US Prescribing Information, Parke-Davis, 1991
Baker MA. The management of leukaemia in the elderly. Balliere’s Clinical Haematology 1: 427–448, 1987
Begleiter A, Glazer RI, Israels LG, Pugh L, Johnston JB. Induction of DNA strand breaks in chronic lymphocytic leukemia following treatment with 2′-deoxycoformycin in vivo and in vitro. Cancer Research 47: 2498–22503, 1987
Begleiter A, Verburg L, Israels LG, Johnston JB. Factors influencing the inhibition and repair of irradiation-induced DNA damage by 2′-deoxycoformycin and deoxyadenosine. Cancer Chemotherapy and Pharmacology 30: 65–69, 1992
Black DJ, Livingston RB. Antineoplastic drugs in 1990. Drugs 39: 489–501, 1990
Blick M, Lepe-Zuniga JL, Doig R, Quesada JR. Durable complete remissions after 2′-deoxycoformycin treatment in patients with hairy cell leukemia resistant to interferon alpha. American Journal of Hematology 33: 205–209, 1990
Bouza E, Burgaleta C, Golde DW. Infection in hairy cell leukemia. Blood 51: 851–859, 1978
Bouroncle BA, Grever MR, Kraut EH. Treatment of hairy cell leukemia: The Ohio State University experience with deoxycoformycin. Leukemia 1: 350–354, 1987
Brady TG. Adenosine deaminase. Biochemical Journal 36:478, 1942
Brady TG, O’Donovan CI. A study of tissue distribution of adenosine deaminase in six mammal species. Comparative Biochemistry and Physiology 14: 101, 1965
Brito-Babapulle F, Huang D, Lavender P, Galton D, Catovsky D. Regression of intracerebral lesions in T prolymphocytic leukaemia treated with intravenous deoxycoformycin. European Journal of Haematology 40: 185–187, 1988
Brox L, Ng A, Pollock E, Belch A. DNA strand breaks induced in human T-lymphocytes by the combination of deoxyadenosine and deoxycoformycin. Cancer Research 44: 934–937, 1984
Brox LW, Pollock E, Belch A. Adenosine and deoxyadenosine toxicity in colony assay systems for human T-lymphocytes, B-lymphocytes, and granulocytes. Cancer Chemotherapy and Pharmacology 9: 49–52, 1982
Bryson HM, Sorkin EM. Cladribine. A review of its pharmacodynamics, pharmacokinetics and therapeutic potential in the treatment of haematological emergencies. Drugs 46: in press, 1993
Bunn PA, Schecter GP, Jaffe E. Clinical course of retrovirus-associated adult T-cell lymphoma in the United States. New England Journal of Medicine 309: 257–264, 1983
Burridge PW, Paetku V, Henderson JF. Studies on the relationship between adenosine deaminase and immune function. Journal of Immunology 119: 675–678, 1977
Camisa C, Grever MR, Bouroncle B. Deoxycoformycin: A new chemotherapeutic agent of interest to dermatologists. Journal of the American Academy of Dermatology 12: 1108–1109, 1985
Carson DA, Iizasa T, Seto S. Metabolic basis of immune dysfunction in adenosine deficiency. Annals of the New York Academy of Sciences 251: 34–42, 1985
Carson DA, Wasson DB, Lakow E, Kamatani N. Possible metabolic basis for the different immunodeficient states associated with genetic deficiencies of adenosine deaminase and purine nucleoside phosphorylase. Proceedings of the National Academy of Sciences of the United States of America 79: 3848–3852, 1982
Cass CE, Seiner M, Tan TH, Muhs WH, Robins MJ. Comparison of the effects on cultured L1210 leukemia cells of the Ribosyl, 2′-deoxyribosyl, and xylosyl homologs of tubercidin and adenosine alone or in combination with 2′-deoxycoformycin. Cancer Treatment Reports 66: 317–326, 1982
Cassileth PA, Cheuvart B, Speirs ASD, Harrington DP, Cummings FJ, et al. Pentostatin induces durable remissions in hairy cell leukemia. Journal of Clinical Oncology 9: 243–246, 1991
Catovsky D. Hairy cell leukemia and prolymphocytic leukemia. Clinical Haematology 6: 245–268, 1977
Catovsky D. Greaves MF, Rose M, Galton DAG, Goolden AWG, et al. Adult T-cell lymphoma-leukaemia in blacks from the West Indies Lancet 1: 639–643, 1982
Chassin MM, Adamson H, Zharevitz W. Enzyme inhibition titration assay for 2′-deoxycoformycin and its application to the study of the relationship between drug concentration and tissue adenosine deaminase in dogs and rats. Biochemical Pharmacology 28: 1849–1855, 1979
Cheson BD. Study design in chronic lymphocytic leukemia. Nouvelle Revue Française Hematologie 30: 407–410, 1988
Coleman MS, Danton MJ, Philips A. Adenosine deaminase and immune dysfunction. Annals of the New York Academy of Sciences 451: 54–65, 1985
Copelan EA, Johnson SC, Grever MR, Sheridan JF, Tutschka PJ. Pharmacological bone marrow purging in murine T cell leukemia. Blood 71: 1656–1661, 1988
Cummings FJ, Crabtree GW, Wieman MC, Spremulli EN, Parks RE, et al. Clinical, pharmacologic, and immunologic effects of 2′-deoxycoformycin. Clinical Pharmacology and Therapeutics 44: 501–509, 1988
Cummings FJ, Kim K, Neiman RS, Comis RL, Oken MM, et al. Phase II trial of pentostatin in refractory lymphomas and cutaneous T-cell disease. Journal of Clinical Oncology 9: 565–571, 1991
Daenen S, Rojer RA, Smit JW, Halie MR, Nieweg HO. Successful chemotherapy with deoxycoformycin in adult T-cell lymphoma-leukaaemia. British Journal of Haematology 58: 723–727, 1984
Dang-Vu AP, Olsen EA, Vollmer RT, Greenberg ML, Hershfield MS. Treatment of cutaneous T cell lymphoma with 2′-deoxycoformycin (pentostatin). Journal of the American Academy of Dermatology 19: 692–698, 1988
Dearden C, Catovsky D. Treatment of hairy cell leukaemia with 2′-deoxycoformycin. Leukemia and Lymphoma 1: 179–185, 1990
Dearden C, Matutes E, Catovsky D. Deoxycoformycin in the treatment of mature T-cell leukaemias. British Journal of Cancer 64: 903–906, 1991
Dearden CE, Matutes E, Hoffbrand AV, Ganeshaguru K, Brozovic M, et al. Membrane phenotype and response to deoxycoformycin in mature T cell malignancies. British Medical Journal 295: 873–875, 1987
Delai BI, Freier L, Johnston JB, Merry CC, Israels LG. Peripheral blood and bone marrow changes following 2′-deoxycoformycin therapy in hairy cell leukemia. Cancer 63: 14–22, 1989
Denz H, Nachbaur D, Lechleitner M, Thaler J, Huber H. Effect of human recombinant interferon (IFN) in combination with deoxycoformycin (2′-deoxycoformycin) on human tumor cell lines. Abstract I-66. Journal of Interferon Research 7: 740, 1987
Dillman RO, Mick R, Mclntyre OR. Pentostatin in chronic lymphocytic leukemia. A phase II trial of Cancer and Leukemia Group B. Journal of Clinical Oncology 7: 433–438, 1989
Dönner H, Ho AD, Stryckmans P, Lauria F, Lechner K, et al. Pentostatin in prolymphocytic leukemia: A phase II trial of the EORTC Leukemia Study Group. Abstract 81. Onkologie 14: 29, 1991
Duggan DB, Anderson JR, Dillman R, Case D, Gottlieb AJ. 2′-deoxycoformycin (pentostatin) for refractory non-Hodgkin’s lymphoma: A CALGB phase II study. Medical and Pediatric Oncology 18: 203–206, 1990
Epstein RB, Fey T, Sarpel S. Prolongation of canine skin graft survival by deoxycoformycin. Transplantation 33: 208–209, 1982
Fabian I, Williams Z. The effect of deoxycoformycin on bone marrow cells treated with adenosine and deoxyadenosine and hemopoietic growth factors. Human Immunology 21: 81–87, 1988
Foon KA, Rai KR, Gale RP. Chronic lymphocytic leukemia: New insights into biology and therapy. Annals of Internal Medicine 113: 525–539, 1990
Faltynek CR. Opposite effects of recombinant interferon-αA and deoxycoformycin on adenosine deaminase activity in the Daudi B lymphoblastoid cell line. Blood 71: 59–64, 1988
Foss FM, Ihde DC, Breneman DL, Phelps RM, Fischmann AB, et al. Phase II study of pentostatin and intermittent high-dose recombinant interferon alfa-2a in advanced mycosis fungoides/Sezary syndrome. Journal of Clinical Oncology 10: 1907–1913, 1992
Galton CAG, Goldman JM, Wiltshaw W, Catovsky E, Henry K, et al. Prolymphocytic leukaemia. British Journal of Haematology 27: 7–23, 1974
Ganeshaguru K, De Mel WCP, Sissolak D, Dearden CE, Mehta AB, et al. Increase in 2′,5′-oligodenylate synthetase caused by deoxycoformycin in hairy cell leukaemia. British Journal of Haematology 80: 194–198, 1992
Ganeshaguru K, No AD, Piga A, Catovsky D, Hoffbrand V. Biochemical mechanisms of deoxycoformycin toxicity in chronic leukemias. Leukemia Research 11: 941–945, 1987
Geiger JD, Lewis JL, MacIntyre CJ, Nagy JI. Pharmacokinetics of 2′-deoxycoformycin, an inhibitor of adenosine deaminase, in the rat. Neuropharmacology 26: 1383–1387, 1987
Giblett ER, Anderson JE, Cohen F, Pollara B, Meuwissen HJ. Adensoine deaminase deficiency in two patients with severely impaired celllular immunity. Lancet 2: 1069–1069, 1972
Goday A, Simmonds HA, Morris GS, Fairbanks LD. Human B lymphocytes and thymocytes but not peripheral blood mononuclear cells accumulate high dATP levels in conditions simulating ADA deficiency. Biochemical Pharmacology 34; 3561–3569, 1985
Golomb HM. Hairy cell leukemia: An unusual lymphoproliferative disease. Cancer 42: 946–956, 1978
Golomb HM, Hanauer SB. Infectious complications associated with hairy cell leukemia. Journal of Infectious Diseases 143: 639–643, 1981
Golomb HM, Mintz V. Treatment of hairy cell leukemia II, chlorambucil therapy in post splenectomy patients with progressive disease. Blood 54: 305–310, 1979
Gray DP, Grever MR, Siaw MFE, Coleman MS, Balcerzak SP. 2′-deoxycoformycin and 9-β-D-arabinofuranosyladenine (AraA) in the treatment of refractory acute myelocytic leukemia. Cancer Treatment Reports 66: 253–257, 1982
Grem JL, King SA, Cheson BD, Leyland-Jones B, Wittes RE. Pentostatin in hairy cell leukemia: Treatment by the special exception mechanism. Journal of the National Cancer Institute 81: 48–53, 1989
Grem JL, King SA, Chun Hg, Grever MR. Cardiac complications observed in elderly patients following 2′-deoxycoformycin therapy. American Journal of Haematology 38: 245–247, 1991
Grever M, Kopecky K, Head D, Cassileth P, Golomb H, et al. A randomized comparison of deoxycoformycin versus alpha-2a interferon in previously untreated patients with hairy cell leukemia: An NCI sponsored intergroup study (SWOG, ECOG, CALGB, NCIC CTG). Abstract 868. Proceedings of American Society of Clinical Oncology 11: 264, 1992
Grever MR, Leiby JM, Kraut EH, Wilson HE, Neidhart JA, et al. Low-dose deoxycoformycin in lymphoid malignancy. Journal of Clinical Oncology 3: 1196–1201, 1985
Grever MR, Staubus AE, Balcerzak SP, Kraut EH, Malspeis L. Clinical pharmacokinetics of 2’-deoxycoformycin in renal impairment. Abstract 361. American Society of Clinical Oncology 12, March 1993
Habermann TM, Andersen JW, Cassileth PA, Bennett JM, Oken MM. Sequential administration of recombinant interferon alpha and deoxycoformycin in the treatment of hairy cell leukaemia. British Journal of Haematology 80: 466–471, 1992
Hacker B, Chang Y. Enhancement of the antitumor activity of N 6-(Δ2-isopentenyl) adenosine against cultured L-12110 leukemia cells by pentostatin using a polymeric delivery system Journal of Pharmaceutical Sciences 72: 902–905, 1983a
Hacker B, Chang Y. Increased cytoxicity of N 6-(Δ2-isopentenyl)adenosine in combination with pentostatin against leukemia cells. Journal of Pharmaceutical Sciences 72: 1225–1226, 1983b
Hallam LJ, Van der Weyden MB, Ackland SP, Bagnara AS, Whiteside MG. The biochemical and clinical consequences of 2′-deoxycoformycin in T cell chronic lymphocytic leukaemia. Scandinavian Journal of Haematology 32: 55–64, 1984
Hershfield MS, Kredich NM. S-adenosylhomocysteine hydrolase is an adenosine-binding protein: a target for adenosine toxicity. Science 202: 757–760, 1978
Ho AD. Chemotherapy of chronic haematological malignancies. Bailliere’s Clinical Haematology 4: 197–221, 1991
Ho AD, Ganeshaguru K, Knauf WU, Dietz G, Trede I, et al. Clinical response to deoxycoformycin in chronic lymphoid neoplasms and biochemical changes in circulating malignant cells in vivo. Blood 72: 1884–1890, 1988
Ho AD, Döhner H, Thaler J, Stryckmans P, Soneveld P, et al. Pentostatin in prolymphocytic leukemia, a phase II study of the EORTC Leukemia Cooperative Group. Abstract 888. Proceedings of American Society of Clinical Oncology 11: 269, 1992b
Ho AD, Klotzbücher A, Gross A, Dietz G, Mestan J, et al. Induction of intracellular and plasma 2′,5′-oligoadenylate synthetase by pentostatin. Leukemia 6: 209–214, 1992a
Ho AD, Männel DN, Wulf G, Kirchner H, Gundt A, et al. Long term effects of 2′-deoxycoformycin treatment on cytokine production in patients with hairy cell leukemia. Leukemia 4: 584–589, 1990a
Ho DHW, Pincus C, Carter CJ, Benjamin RS, Freireich EJ, et al. Distribution and inhibition of adenosine deaminase in tissues of man, rat, and mouse. Cancer Treatment Reports 64: 629–633, 1980
Ho AD, Thaler J, Mandelli F, Lauria F, Zittoun R, et al. Response to pentostatin in hairy cell leukemia refractory to interferon-alpha. Journal of Clinical Oncology 7: 1533–1538, 1989
Ho AD, Thaler J, Stryckmans P, Coiffer B, Luciani K, et al. Pentostatin in refractory chronic lymphocytic leukemia: A phase II trial of the European Organisation for Research and Treatment of Cancer. Journal of the National Cancer Institute 82: 1416–1420, 1990b
Ho AD, Thaler J, Willemze R, Lauria F, Mandelli F, et al. Pentostatin (2′-deoxycoformycin) for the treatment of lymphoid neoplasms. Cancer Treatment Reviews: 213–215, 1990c
Holloway KB, Flowers FP, Ramos-Caro F. Therapeutic alternatives in cutaneous T-cell lymphoma. Journal of the American Academy of Dermatology 27: 367–378, 1992
Hovi T, Smyth JF, Allison AC, Williams SC. Role of adenosine deaminase in lymphocyte proliferation. Clinical and Experimental Immunology 23: 395, 1976
Johnston JB, Eisenhauer E, Corbett WEN, Scott G, Zaentz SD. Efficacy of 2′-deoxycoformycin in hairy cell leukemia: A study of the National Cancer Institute of Canada Clinical Trials Group. Journal of the National Cancer Institute 80: 765–769, 1988
Johnston JB, Glazer RI, Pugh L, Israels LG. The treatment of hairy-cell leukaemia with 2′-deoxycoformycin. British Journal of Haematology 63: 525–534, 1986
Johnston JB, Lee K, Verburg L, Blondal J, Mowat MRA, et al. Induction of apoptosis in CD4+ prolymphocytic leukaemia by deoxyadenosine and 2′-deoxycoformycin. Leukemia Research 16: 781–788, 1992
Kefford RF, Fox RM. Purine deoxynucleoside toxicity in nondividing human lymphoid cells. Cancer Research 42: 324–330, 1982
Kizaki H, Shimada H, Ohsaka F, Sakurada T. Adenosine, deoxydenosine, and deoxyguanosine induce DNA cleavage in mouse thymocytes. Journal of Immunology 141: 1652–1657, 1988
Kraut EH, Bouroncle BA, Grever MR. Pentostatin in the treatment of advanced hairy cell leukemia. Journal of Clinical Oncology 7: 168–172, 1989
Kraut EH, Grever MR, Gochnour D, Bouroncle BA. Long term followup of hairy cell leukemia patients after 2′-deoxycoformycin. Abstract 876. Proceedings of American Society of Clinical Oncology 11: 266, 1992
Kraut EH, Neff JC, Bouroncle BA, Gochour D, Grever MR. Immunosuppressive effects of pentostatin. Journal of Clinical Oncology 8: 848–855, 1990
Lahat N, Aghai E, Merchav S, Kinarty A, Sobel E, et al. Concomitant effect of 2′-deoxycoformycin on natural killer cell activity and tumour cell sensitivity to lysis in hairy cell leukaemia — discordant effects of alpha interferon. Scandinavian Journal of Immunology 32: 205–209, 1990
Lamballe F, Le Prise P-Y, Le Gall E, David J-C. dATP-mediated inhibition of DNA ligase by 2′-deoxycoformycin in T and B cell leukemia. Leukemia 3: 97–103, 1989
Lee SH, Thomas LK, Unger FM, Christian R, Sartorelli AC. Comparative antineoplastic activity against P3889 leukemia of 9-β-D-arabinofuranosyladenine (araA) and 9-β-(2′-azido-2′-deoxy-D-arabinofuranosyl)adenine (arazide). International Journal of Cancer 27:703–708, 1981
Lindley CM, Bernard SA, Robertson JD. Vomiting associated with pentostatin and pentostatin plus alpha-interferon: Unique pattern and potential mechanisms. Journal of Pain and Symptom Management 5: 262–264, 1990
Lofters W, Campbell M, Gibbs WN, Cheson BD. 2′-deoxycoformycin therapy in adult T-cell leukemia/lymphoma. Cancer 60: 2605–2608, 1987
Lum CT. Sutherland DER, Payne WD, Gorecki P, Matus AJ, et al. Prolongation of mouse and rat pancreatic islet allografts by adenosine deaminase inhibitors and adenine arabinoside. Journal of Surgical Research 28: 44–48, 1980
Major PP, Agarwal RP, Kufe DW. Clinical pharmacology of deoxycoformycin. Blood 58: 91–96, 1981a
Major PP, Agarwal RP, Kufe DW. Deoxycoformycin: neurologiical toxicity. Cancer Chemotherapy and Pharmacology 5: 193–196, 1981b
Malspeis L, Weinrib AB, Straubus AE, Grever MR, Balcerzak SP, et al. Clinical Pharmacokinetics of 2′-deoxycoformycin. Cancer Treatment Symposia 2: 7–15, 1984
Martin A, Nerenstone S, Urba WJ, Longo DL, Lawrence JB, et al. Treatment of hairy cell leukemia with alternating cycles of pentostatin and recombinant leukocyte A interferon: Results of a phase II study. Journal of Clinical Oncology 8: 721–730, 1990
Matsumoto SS, Yu J, Yu AL. Inhibition of RNA synthesis by deoxyadenosine plus deoxycoformycin in resting lymphocytes. Journal of Immunology 131: 2762–2766, 1983
Matsumoto SS, Yu J, Yu AL. The effect of deoxyadenosine plus deoxycoformycin on replicative and repair synthesis of DNA in human lymphoblasts and isolated nuclei. Journal of Biological Chemistry 263: 7153–7158, 1988
Matutes E, Brito-Babapulle V, Swansbury J, Ellis J, Morilla R, et al. Clinical and laboratory features of 78 cases of T-prolymphocytic leukemia. Blood 78: 3269–3274, 1991
Miser JS, Roloff J, Blatt J, Reaman GH, Kraialo MD, et al. Lack of significant activity of 2′-deoxycoformycin alone or in combination with adenine arabinoside in relapsed childhood acute lymphoblastic leukemia. American Journal of Oncology 15: 490–493, 1992
Mitchell, BS, Sidi Y, Hershfield M, Koller CA. Biochemical consequences of adenosine deaminase inhibition in vivo. Annals of the New York Academy of Sciences 451: 129–137, 1985
Montserrat E, Lozano M, Urbano-Ispizua A, Matutes E, Feliu E, et al. Adult T-cell leukemia in a Chilean resident in Spain: Long-lasting remission after 2′-deoxycoformycin treatment. Leukemia and Lymphoma 1: 47–49, 1989
Murphy SB, Herrod HG, Sinkule JA, Abromowitch M, Rivera G. Severe combined immunodeficiency and profound weight loss associated with chronic intermittent administration of 2′-deoxycoformycin. Cancer Treatment Symposia 2: 63–66, 1984a
Murphy SB, Sinkule JA, Rivera G. Phase I-II clinical and pharmacodynamic study of effects of 2′-deoxycoformycin administered by continuous infusion in children with refractory acute lymphoblastic leukemia. Cancer Treatment Symposia 2: 55–61, 1984b
O’Dwyer PJ, King SA, Eisenhauer E, Grem JL, Hoth DF. Hypersensitivity reactions to deoxycoformycin. Cancer Chemotherapy and Pharmacology 23: 173–175, 1989
O’Dwyer PJ, Speirs AS, Marsoni S. Association of severe and fatal infections and treatment with pentostatin. Cancer Treatment Reports 70: 1117–1120, 1986
O’Dwyer PJ, Wagner B, Leyland-Jones B, Wittes RE, Cheson BD, et al. 2′-Deoxycoformycin (pentostatin) for lymphoid malignancies. Rational development of an active new drug. Annals of Internal Medicine 108: 733–743, 1988
Padua RA. Geiger JD, Delany SM, Nagy JL Rat brain adenosine deaminase after 2′-deoxycoformycin administration: Biochemical properties and evidence for reduced enzyme levels detected by 2′-[H3]deoxycoformycin ligand binding. Journal of Neurochemistry 58: 421–429, 1992
Pentostatin (Nipent™) prescribing information, Parke-Davis, Division of Warner-Lambert Co, New Jersey, USA, Oct, 1991
Piga Am, Ganeshaguru K, Green ES, Sheridan B, Hoffbrand AV. Selective toxicity of purine nucleosides to human leukaemic cells. Advances in Experimental and Medical Biology 253: 291–298, 1989
Plunkett W, Nowak B, Feun LG, Benjamin RS, Keating M, et al. Modulation of arabinosyladenine metabolism by 2′-deoxycoformycin in the therapy of human leukemia. Advances in Experimental Medicine and Biology 165: 345–350, 1984
Poplack DG, Sallan SE, Rivera G, Holcenberg J, Murphy SB, et al. Phase I study of 2′-deoxycoformycin in acute lymphoblastic leukaemia. Cancer Research 41: 3343–3346, 1981
Prentice HG, Lee N, Blacklock H, Smyth JF, Russell NH, et al. Therapeutic selectivity of and prediction of response to 2′-deoxycoformycin in acute leukaemia. Lancet 2: 1250–1253. 1981
Prentice HG, Ganeshaguru K, Bradstock KF, Goldstone AH, Smyth KF, et al. Remission induction with adenosine deaminase inhibitor 2′-deoxycoformycin in Thy-lymphoblastic leukaemia. Lancet 2: 170–172, 1980
Ratain MJ, Vardiman JW, Barker CM, Golomb HM. Prognostic variables in hairy cell leukemia after splenectomy as initial therapy. Cancer 62: 2420–2424, 1988
Ratech H, Kim J, Asofsky R, Thorbecke J, Hirschhorn R. Effects of deoxycoformycin in mice. II. Differences between the drug sensitivities and purine metabolizing enzymes of transplantable lymphomas of varying immunologic phenotypes. The Journal of Immunology 132: 3077–3084, 1984
Ratech H, Thorbecke J, Hirschhorn R. Metabolic abnormalities of human adenosine deaminase deficiency reproduced in the mouse by 2′-deoxycoformycin, an adenosine deaminase inhibitor. Clinical Immunology and Immunopathology 21: 119–127, 1981
Ross SR, McTavish D, Faulds D. Fludarabine. A review of its pharmacological properties and therapeutic potential in malignancy. Drugs 45: 737–759, 1993
Ruers TJM, Buurman WA, Van der Linden CJ, Kootstra G. Complete suppression of skin allograft rejection in rats treated with continuous infusion of 2′-deoxycoformycin. Transplantation 40: 137–142, 1985
Seto S, Carrera CJ, Kubota M, Wasson DB. Mechanism of deoxyadenosine and 2-chlorodeoxyadenosine toxicity to non-dividing human lymphocytes. Journal of Clinical Investigation 75: 377–383, 1985
Shimoyama M. Treatment of patients with adult T-cell leukemialymphoma: An overview. In Gann Monograph on Cancer Research 39, pp 43–56, Japan Scientific Press, Tokyo, 1992
Siaw MFE, Coleman MS. In vitro metabolism of deoxycoformycin in human T lymphoblastoid cells. Journal of Biological Biochemistry 259: 9426–9433, 1984
Sordillo EM, Ikehara S, Good RA, Trotta PP. Immunosuppression by 2′-deoxycoformycin: Studies on the mode of administration. Cellular Immunology 63: 259–271, 1981
Sorensen JM, Chun HG, Vena D, Fallavollita A, Cheson BD. Pentostatin (2′-deoxycoformycin) therapy for hairy cell leukemia: Update of a group C protocol of 208 patients who have failed interferon alpha. Abstract 787. Proceedings of American Society of Clinical Oncology 10: 232, 1991
Smyth JF, Harrap KR. Adenosine deaminase activity in leukaemia. British Journal of Cancer 31: 544–549, 1975
Smyth JF, Paine RM, Jackman AL, Harrap KR, Chassin MM, et al. The clinical pharmacology of adenosine deaminase inhibitor 2′-deoxycoformycin. Cancer Chemotherapy and Pharmacology 5: 93–101 1980
Speirs ASD, Davis MP, Levine M, Li CY, Mangan KF, et al. T-Cell chronic lymphocytic leukemia: Anomalous cell markers, variable morphology, and marked responsiveness to pentostatin (2′-deoxycoformycin). Scandinavian Journal of Haematology 34: 57–67, 1985
Speirs ASD, Moore D, Cassileth PA, Harrington DP, Cummings FJ, et al. Remissions in hairy cell leukemia with pentostatin (2′-deoxycoformycin). New England Journal of Medicine 316: 825–830, 1987
Speirs ASD, Parekh SJ. The treatment of hairy cell leukemia with 2′-deoxycoformycin: Results in India and in the United States. Leukemia 1: 347–350, 1987
Speirs AS, Parekh SJ, Bishop MB. Hairy-cell leukaemia: induction of complete remission with pentostatin (2′-deoxycoforomycin). Journal of Clinical Oncology 2: 1336–1342, 1984
Speirs ASD, Ruckdeschel JC, Horton J. Effectiveness of pentostatin (2′-deoxycoformycin) in refractory lymphoid neoplasms. Scandinavian Journal of Haematology 32: 130–134, 1984
Steinmetz JC, DeConti R, Ginssburg R. Hypersensitivity vasculitis associated with 2-deoxycoformycin and allopurinol therapy. American Journal of Medicine 86: 499, 1989
Steis RG, Urba WJ, Kopp WC, Alvord WG, Smith JW, et al. Kinetics of recovery of CD4+ T cells on peripheral blood of deoxycoformycin-treated patients. Journal of The National Cancer Institute 83: 16788–1679, 1991
Straubus AE, Weinrib AB, Malspeis L. An enzymatic kinetic method for the determination of 2′-deoxycoformycin in biological fluids. Biochemical Pharmacology 33: 1633–1635, 1984
Svendsen KR, Overgaard-Hansen K, Frederiksen S. Synergistic Effect of 3′-deoxyadenosine N 1-oxide and adenosine deaminase inhibitors on growth of Ehrlich ascites tumor cells in vivo. Cancer Chemotherapy and Pharmacology 21:35–39, 1988
Sylwestrowitz TA, Ma DDF, Murphey PP, Massaia M, Prentice HG, et al. 5′-Nucleotidase, adenosine deaminase and purine nucleoside phosphorylase activities in acute leukaemia. Leukemia Research 6: 475–482, 1982
Tanaka M, Kimura K. Differential sensitivity of leukemic cells to growth inhibition by deoxycoformycin. Tohoku Journal of Experimental Medicine 147: 331–341, 1985
Tedde A, Balis ME, Ikehara S, Pahwa R, Good RA, et al. Animal model for immune dysfunction associated with adenosine deficiency. Proceedings of the National Academy of the Sciences of the United States of America 77; 4899–4903, 1980
Thaler J. Grünewald K, Gattringer C, Ho AD, Weyrer K, et al. Long-term follow-up of patients with hairy cell leukaemia treated with pentostatin: lymphocyte subpopulations and residual marrow infiltration. Bristish Journal of Haematology 84: 75–82, 1993
Thliveris JA, Begleiter A, Kobrinsky NL, Verburg L, Dean HJ, et al. Prevention of insulin-dependent diabetes mellitus by 2′-deoxycoformycin in the BB wistar rat. Biochemical Pharmacology, in press, 1993
Tobinai K, Shimoyama M, Inoue S, Takayasu S, Mikuni C, et al. Phase I study of YK-176 (2′-deoxycoformycin) in patients with adult T-cell leukemia/lymphoma. Japanese Journal of Clinical Oncology 22: 164–171, 1992
Trotta PP, Tedde A, Ikehara S, Pahwa R, Good RA, et al. Specific immunosuppressive effect of constant infusion of 2′-deoxycoformycin. Cancer Research 41: 2189–2196, 1981
Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukaemia: Clinical and hematological features of 16 cases. Blood 50: 481–492, 1977
Ullman B, Levinson BB, Hershfield MS, Martin DW. A biochemical study of the role of specific nucleoside kinases in deoxyadenosine phosphorylation by cultured human cells. Journal of Biological Chemistry 256: 848–852, 1981
Urba WJ, Baseler MW, Kopp WC, Steis RG, Clark JW, et al. Deoxycoformycin-induced immunosuppression in patients with hairy cell leukemia. Blood 73: 38–46, 1989
Venner PM, Glazer RI, Blatt J, Sallan S, Rivera G, et al. Levels of 2′-deoxycoformycin and deoxyadenosine in patients with acute lymphoblastic leukemia. Cancer Research 41: 4508–4511, 1981
Weitberg AB, Corvese D. Niacin prevents DNA strand breakage by adenosine deaminase inhibitors. Biochemical and Biophysical Research Communications 167: 514–519, 1990
Winick N, Buchanan GR, Murphy SB, Yu A, Boyett J. Deoxycoformycin treatment for childhood T-cell acute lymphoblastic leukemia early in second remission: A Pediatric Oncology Group Study. Medical and Pediatric Oncology 16: 327–332, 1988
Yamaguchi K, Yul LS, Oda T, Maeda Y, Ishii M, et al. Clinical consequences of 2′-deoxycoformycin treatment in patients with refractory adult T-cell leukaemia. Leukemia Research 10: 989–993, 1986
Yu AL, Kung FH, Bakay B, Nyhan WL. In vitro and in vivo effect of deoxycoformycin in human T cell leukemia, advances in Experimental Medicine and Biology 122: 373–379, 1979
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: A. Begleiter, Manitoba Institute of Cell Biology, Winnipeg, Manitoba, Canada; F.J. Cummings, Roger Williams General Hospital, Providence, Rhode Island, USA; F. Foa, Dipartmento di Scienze Biomediche e Oncologia Umana, Universita Torino, Torino, Italy; K. Ganeshaguru, Department of Haematology, Royal Free Hospital of Medicine, Hampstead, London, England; A.V. Hoffbrand, Department of Haematology, Royal Free Hospital of Medicine, Hampstead, London, England; G. Juliusson, Karolinska Institute, Division of Hematology and Oncology, Huddinge University Hospital, Huddinge, Sweden; E.H. Kraut, Division of Hematology and Oncology, Arthur G. James Cancer Hospital and Research Institute, Columbus, Ohio, USA; F. Lauria, Istituto di Ematologia, Lorenzo e Ariosto Seràgnoli, Bologna, Italy; C. Sundström, Department of Pathology, Hemopathology Section, University Hospital Uppsala, Uppsala, Sweden; K. Tobinai, Department of Medical Oncology, National Cancer Center Hospital, Tokyo, Japan.
Rights and permissions
About this article
Cite this article
Brogden, R.N., Sorkin, E.M. Pentostatin. Drugs 46, 652–677 (1993). https://doi.org/10.2165/00003495-199346040-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199346040-00006