Summary
Synopsis
Coronary arterial thrombolysis is becoming an established treatment of acute myocardial infarction. If given early enough, it recanalises occluded coronary arteries, salvages myocardial function and reduces mortality. A reduction of mortality in patients with acute myocardial infarction has now been demonstrated for streptokinase, anisoylated plasminogen streptokinase activator complex (APSAC; anistreplase) and recombinant tissue-type plasminogen activator (rt-PA)
From the biochemical point of view, rt-PA has several attractive properties. It is similar to or identical with the physiological plasminogen activator in blood, it does not induce an antibody response, and it is more fibrin-specific than most or all other currently known thrombolytic agents. The rate of recanalisation of occluded coronary arteries with rt-PA is about 60 to 80% in non-comparative and placebo-controlled trials. rt-PA was similar in efficacy to urokinase in the only trial to compare the 2 agents. In 2 comparative trials evaluated by meta-analysis, rt-PA appeared more effective than streptokinase for the early recanalisation of occluded arteries. Both agents were comparable in their effects on left ventricular function in 2 comparative trials, but further study is needed to conclusively evaluate this parameter. Moreover, both agents reduce inhospital mortality, but much larger direct comparative trials are required before scientifically valid statements can be made on the relative clinical efficacy of available thrombolytic agents in terms of their effects on both morbidity and mortality.
Thus, rt-PA constitutes a notable contribution of recombinant DNA technology to the treatment of thromboembolic disease, the main cause of death and disability in Western societies.
Pharmacodynamic Properties
Recombinant tissue-type plasminogen activator (rt-PA) for clinical use is produced by bulk fermentation of a Chinese hamster ovary cell line transfected with the cDNA for the naturally occurring human product. The subsequently purified proteinase is a single polypeptide chain of 527 amino acids which is fully glycosylated and identical to the naturally occurring human protein.
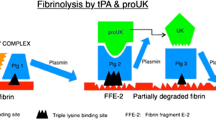
The mechanism of action of rt-PA is similar to that of naturally occurring t-PA. t-PA has a high affinity to fibrin in a thrombus. In turn, t-PA has a high affinity and specificity towards fibrin-bound plasminogen, where it causes enzymatic degradation of the latter into plasmin and consequently thrombolysis. t-PA has only low affinity for plasminogen in the absence of fibrin. Thus, the fibrinolytic process induced by t-PA is fibrin-specific and causes only limited systemic plasminogen activation and fibrinogenolysis.
Various in vitro studies have demonstrated the fibrinolytic activity of t-PA against clots, while causing only minor systemic activation of the fibrinolytic system. The in vivo thrombolytic properties of t-PA have been confirmed in numerous and varied animal models of thrombolysis, including pulmonary emboli, thrombosis of jugular and femoral veins and coronary and femoral arteries. t-PA was more potent than u-PA (urokinase-type plasminogen activator), and it produced more rapid and more effective lysis. It was also more rapid and more effective than streptokinase. In addition, t-PA caused less extensive systemic breakdown of fibrinogen than u-PA and streptokinase. Preliminary animal studies suggest a use for t-PA in stroke and some ophthalmological conditions.
Pharmacokinetic Properties
The disposition of t-PA in plasma can be represented by a 2-compartment model composed of 1 central (plasma) compartment and 1 peripheral compartment. t-PA is rapidly cleared from circulation by the liver, with an initial half-life of only a few minutes in animals. However, fibrin-bound t-PA remains pharmacologically active at the clot site for several hours after withdrawal of the systemic infusion of rt-PA and its clearance from circulation. In healthy subjects intravenous infusion of commercially available rt-PA (8.3 μg/kg/min) yields a mean steady-state plasma concentration of about 1 to 1,5 mg/L. Other mean pharmacokinetic parameters in subjects were: initial half-life 3 to 4 minutes; terminal half-life about 30 minutes; plasma clearance about 40 L/h; and volumes of distribution of the central compartment and at steady-state of 3.9 and 7.2L, respectively. Estimation of t-PA may vary significantly depending on the assay system used and on the method of blood collection and storage.
Therapeutic Studies
Using coronary artery reperfusion or patency as end-points, intravenous infusion of rt-PA is superior to placebo in the treatment of acute myocardial infarction. At total doses of 40 to 100mg administered over 1 to 3 hours, rt-PA produces reperfusion in about 60 to 80% of infarct-related arteries, as demonstrated in non-comparative trials. Compared with placebo, rt-PA improves regional wall motion of the infarcted zone and enhances left ventricular function. The earlier rt-PA is administered after the onset of symptoms the better the chance of reperfusion and salvage of left ventricular function. Overall inhospital mortality after rt-PA therapy was low (4 to 7%), but there were no control groups in most studies. However, 2 recent placebo-controlled studies have shown that mortality is significantly reduced, especially if rt-PA is administered within 3 hours of the onset of symptoms. Reocclusion of the coronary arteries can occur in about 7 to 15% of patients after rt-PA therapy.
Cumulated results of non-comparative patency or reperfusion trials using comparable end-points demonstrate rt-PA to reperfuse coronary arteries more efficiently than streptokinase. Meta-analysis of patency data from 2 trials directly comparing the efficacies of rt-PA and streptokinase indicates a significantly higher frequency of patent arteries with rt-PA at 90 minutes after starting treatment. Reocclusion rates have not been evaluated in directly comparable trials, but appear to be similar. Bleeding complications in comparative trials appear somewhat, but not dramatically, lower with rt-PA than with streptokinase. The relative impact of streptokinase and rt-PA on preservation of left ventricular function has been evaluated in 2 comparative trials, which showed comparable effects for the 2 agents. However, further studies are needed to arrive at conclusive results. In small comparative trials not designed with mortality as end-points, the combined inhospital mortality was 5.4% for rt-PA and 7.7% for streptokinase. Large directly comparative trials designed to specifically address mortality are required to establish the relative impact of each drug on mortality. rt-PA and urokinase were of similar efficacy in the only trial to compare these 2 agents.
Several small studies and case reports have indicated that rt-PA may be useful in the treatment of a variety of other indications including pulmonary embolism, unstable angina pectoris, deep vein thrombosis, peripheral arterial occlusion, stroke and some ophthalmological conditions. However, controlled studies in larger numbers of patients will be required before any definite conclusions can be drawn concerning its efficacy in these indications.
Adverse Effects
Minor adverse effects such as nausea, vomiting, hypotension and fever have been reported with rt-PA, but these effects may not be attributable to the drug as they are frequent sequelae of myocardial infarction. No serious immunogenic reactions have been noted with rt-PA, unlike some other thrombolytic treatments, although mild hypersensitivity reactions such as urticaria have occasionally been observed.
Most interest concerning the tolerability of thrombolytic therapy has centred on the relative risk of systemic fibrinolytic activation and consequent bleeding complications. At usual therapeutic doses rt-PA induces little systemic fibrinolytic activation, in particular compared with streptokinase. However, in clinical practice rt-PA therapy remains associated with a residuai bleeding tendency. The type of bleeding associated with thrombolytic therapy may be divided into 2 kinds: superficial and internal. Superficial bleeding (e.g. venous cutdowns, arterial punctures, sites of recent surgical intervention) are relatively more frequent but not critical compared with the rare, more serious cases of internal bleeding involving the gastrointestinal tract, genitourinary tract, retroperitoneal and intracranial sites. Life-threatening intracranial bleeding occurred in some clinical studies of rt-PA when total doses up to 150mg were used, but there appears to be no increased risk of fatality from this complication since a total dose restriction to a maximum of 100mg has been instituted. In a controlled clinical trial in over 5000 patients who did not receive concomitant aspirin the stroke rates in rt-PA and control groups were similar.
Dosage and Administration
rt-PA is indicated for use in adults with acute myocardial infarction. Treatment should be initiated as soon as possible after the onset of symptoms (at the latest 6 hours after the onset of pain). In the US the recommended total dose is 100mg administered by intravenous infusion as 60mg in the first hour (of which 6 to 10mg is administered as a bolus over the first 1 to 2 minutes) followed by 20mg in each of the subsequent 2 hours. For patients weighing less than 65kg, a total dose of 1.25 mg/kg administered over 3 hours, as described above, may be used. In European countries the recommended total dose is 70 to 100mg in 90 minutes with a 10mg bolus, to a total dosage of 1 mg/kg. Dose regimens adjusted for bodyweight appear to result in an improvement in coronary patency, with a lower rate of haemorrhagic complications. A higher total dose of rt-PA must not be used as 150mg has been associated with an increased risk of intracranial bleeding. In most patients treated to date, heparin has been administered concomitantly for 48 hours or more. Aspirin and/or dipyridamole have been given during and/or following heparin treatment. rt-PA is contraindicated in the following situations: active internal bleeding; history of cerebrovascular accident; intracranial or intraspinal surgery or trauma within 2 months; intracranial neoplasm, arteriovenous aneurysm or malformation; known bleeding diathesis; and severe uncontrolled hypertension. There are several other conditions where the risk of rt-PA therapy may be increased and should be weighed against the anticipated benefits.
Similar content being viewed by others
References
Agnelli G, Buchanan MR, Fernandez F, Boneu B, Van Ryn J, et al. A comparison of the thrombolytic and hemorrhagic effects of tissue-type plasminogen activator and streptokinase in rabbits. Circulation 72: 178–182, 1985b
Agnelli G, Buchanan MR, Fernandez F, Van Ryn J, Hirsh J. Sustained thrombolysis with DNA-recombinant tissue-type plasminogen activator in rabbits. Blood 66: 399–401, 1985a
AIMS Trial Study Group. Effect of intravenous APSAC on mortality after acute myocardial infarction: preliminary report of a placebo-controlled clinical trial. Lancet 1: 545–549, 1988
Alderman EL, Jutzy KR, Berte LE, et al. Randomized comparison of intravenous versus intracoronary streptokinase for myocardial infarction. American Journal of Cardiology 54: 14–19, 1984
Aimér L-O, Öhlin H. Elevated levels of the rapid inhibitor of plaminogen activator in acute myocardial infarction. Thrombosis Research 47: 335–339, 1987
Andreasen PA, Nielsen LS, Grondahl-Hansen J, Skriver L, Zeuthen J, et al. Inactive proenzyme to tissue-type plasminogen activator from human melanoma cells, identified after affinity purification with a monoclonal antibody. EMBO Journal 3: 51–56, 1984
Aznar J, Estellés A, Vila V, Reganon E, Espana F, et al. Inherited fibrinolytic disorder due to an enhanced plasminogen activator level. Thrombosis and Haemostasis 52: 196–200, 1984
Bakhit C, Lewis D, Billings R, Malfroy B. Cellular catabolism of recombinant tissue-type plasminogen activator. Journal of Biological Chemistry 262: 8716–8720, 1987
Bamford J, Sandercock P, Wardlow C. Fatal ischaemic brain oedema after tissue plasminogen activator. Correspondence. British Medical Journal 298: 383, 1989
Banyai L, Varadi A, Patthy L. Common evolutionary origin of the fibrin-binding structures of fibronectin and tissue-type plasminogen activator. FEBS Letters 163: 37–41, 1983
Bebbington CR, Hentschel CC. The expression of recombinant DNA products in mammalian cells. Trends in Biotechnology 3: 314–317, 1985
Beebe DP. Binding of tissue plasminogen activator to human umbilical vein endothelial cells. Thrombosis Research 46: 241–254, 1987
Beebe DP, Aronson DL. Turnover of human tissue plasminogen activator (t-PA) in rabbits. Thrombosis Research 43: 663–674, 1986
Bennett WR, Yawn DH, Migiore PJ, Young JB, Pratt CM, et al. Activation of the complement system by recombinant tissue plasminogen activator. Journal of the American College of Cardiology 10: 627–632, 1987
Bergmann SR, Fox KAA, Ter-Pogassian MM, Sobel B, Collen D. Clot-selective coronary thrombolysis with tissue-type plasminogen activator. Science 220: 1181–1183, 1983
Bertina RM, Van Hinsbergh VWM, Emeis SJ, Van Wijngaarden A. Inhibition of tissue-type plasminogen activator inhibitor activity by activated protein C. Abstract. Circulation 70 (Suppl. II): 94, 1984
Booth NA, Bennett B, Wijngaards G, Grieve JHK. A new lifelong hemorrhagic disorder due to excess plasminogen activator. Blood 61: 267–275, 1983
Booth NA, Walker E, Maughan R, Bennett B. Plasminogen activator in normal subjects after exercise and venous occlusion: t-PA circulates as complexes with C1-inhibitor and PAI-1. Blood 69: 1600–1604, 1987
Bounameaux H, Stassen JM, Seghers C, Collen D. Influence of fibrin and liver blood flow on the turnover and the systemic fibrinogenolytic effects of recombinant human tissue-type plasminogen activator in rabbits. Blood 67: 1493–1497, 1986
Bounameaux H, Vermylen J, Collen D. Thrombolytic treatment with recombinant tissue-type plasminogen activator in a patient with massive pulmonary embolism. Annals of Internal Medicine 103: 64–65, 1985
Braunwald E. Myocardial reperfusion, limitation of infarct size, reduction of left ventricular dysfunction, and improved survival. Should the paradigm be expanded? Circulation 79: 441–444, 1989
Braunwald E, Knatterud GL, Passamani E, Robertson TL. Announcement of protocol change in Thrombolysis in Myocardial Infarction Trial. Journal of the American College of Cardiology 9: 467, 1987a
Braunwald E, Knatterud GL, Passamani E, Robertson TL, Solomon R. Update from the Thrombolysis in Myocardial Infarction Trial. Journal of the American College of Cardiology 10: 970, 1987b
Brochier ML, Quillet L, Kulbertus H, et al. Intravenous anisolylated plasminogen streptokinase activator complex versus intravenous streptokinase in evolving myocardial infarction. Drugs 33 (Suppl. 3): 140–145, 1987
Brott T, Haley EC, Levy DE, Barsan WG, Reed RL, et al. The investigational use of t-PA for stroke. Annals of Emergency Medicine 17: 1202–1205, 1988
Browne MJ, Carey JE, Chapman CG, Tyrrell AWR, Entwisle C, et al. A tissue-type plasminogen activator mutant with prolonged clearance in vivo. Effect of removal of the growth factor domain. Journal of Biological Chemistry 263: 1599–1602, 1988
Browne MJ, Tyrrell AWR, Chapman CG, Carey JE, Glover DM, et al. Isolation of a human tissue-type plasminogen-activator genomic DNA clone and its expression in mouse L cells. Gene 33: 279–284, 1985
Bugelski PJ, Fong K-L, Linkner A, Sowinski J, Rush G, et al. Uptake of human recombinant tissue type plasminogen activator by rat hepatocytes in vivo: an electron microscope autoradiographic study. Thrombosis Research 53: 287–303, 1989
Builder SE, Grossbard E. Laboratory and clinical experience with recombinant plasminogen activator. In Murawski & Peetoom (Eds) Transfusion medicine: recent technological advances, p. 303, A.R. Liss, New York, 1986
Bunning RAD, Crawford A, Richardson HJ, Opdenakker G, Van Damme J, et al. Interleukin 1 preferentially stimulates the production of tissue-type plasminogen activator by human articular chondrocytes. Biochimica et Biophysica Acta 924: 473–482, 1987
Buteux G, Jubault V, Suisse A, Courtheoux P. Local recombinant tissue plasminogen activator to clear cerebral artery thrombosis developing soon after surgery. Lancet 2: 1143–1144, 1988
Califf RM, Topol EJ, George BS, Boswick JM, Abbottsmith C, et al. Hemorrhagic complications associated with the use of intravenous tissue plasminogen activator in treatment of acute myocardial infarction. American Journal of Medicine 85: 353–359, 1988
Cambier P, Van de Werf F, Larsen GR, Collen D. Pharmacokinetics and thrombolytic properties of a nonglycosylated mutant of human tissue-type plasminogen activator, lacking the finger and growth factor domains, in dogs with copper coil-induced coronary artery thrombosis. Journal of Cardiovascular Pharmacology 11: 468–472, 1988
Camiolo SM, Thorsen S, Astrup T. Fibrinogenolysis and fibrinolysis with tissue plasminogen activator, urokinase, streptokinase-activated human globulin, and plasmin. Proceedings of the Society for Experimental Biology and Medicine 138: 277–280, 1971
Carlin G, Einarsson M, Saldeen T. Tissue plasminogen activator effectively lyses intravascular fibrin deposits in the rat lung. In Davidson et al. (Eds) Progress in fibrinolysis, Vol. 6, pp. 471–474, Churchill Livingstone, Edinburgh, 1983
Cash JD. Control mechanism of activator release. In Davidson et al. (Eds) Chemical fibrinolysis and thrombolysis, Vol. 3, pp. 65–75, Raven Press, 1978
Cercek B, Lew AS, Hod H, Yano J, Reddy NKN, et al. Enhancement of thrombolysis with tissue-type plasminogen activator by pretreatment with heparin. Circulation 74: 583–587, 1986
Chesebro JH, Knatternd G, Braunwald E. Thrombolytic therapy. Correspondence. New England Journal of Medicine 319: 1544–1545, 1988
Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Circulation 76: 142–154, 1987
Clayton KJ, Anderson JA, McNicol GP. Preoperative prediction of postoperative deep vein thrombosis. British Medical Journal 2: 910–912, 1976
Collen D, Bounameaux H, De Cock F, Lijnen HR, Verstraete M. Analysis of coagulation and fibrinolysis during intravenous infusion of recombinant human tissue-type plasminogen activator in patients with acute myocardial infarction. Circulation 73: 511–517, 1986a
Collen D, Lijnen HR. The fibrinolytic system in man. CRC Critical Reviews in Hematology and Oncology 4: 249–301, 1986
Collen D, Rijken DC, Van Damme J, Billiau A. Purification of human tissue-type plasminogen activator in centigram quantities from human melanoma cell culture fluid and its conditioning for use in vivo. Thrombosis and Haemostasis 48: 294–296, 1982
Collen D, Stassen JM, Marafino BJ, Buuilder S, De Cock F, et al. Biological properties of deletion mutants of human tissue-type plasminogen activator in rabbits. Blood 71: 216–219, 1988a
Collen D, Stassen JM, Marafino BJ, Builder S, De Cock F, et al. Biological properties of human tissue-type plasminogen activator obtained by expression of recombinant DNA in mammalian cells. Journal of Pharmacology and Experimental Therapeutics 231: 146–152, 1984a
Collen D, Stassen JM, Verstraete M. Thrombolysis with human extrinsic (tissue-type) plasminogen activator in rabbits with experimental jugular vein thrombosis. Effect of molecular form and dose of activator, age of the thrombus, and route of administration. Journal of Clinical Investigation 71: 368–376, 1983
Collen D, Stump DC, Gold HR. Thrombolytic therapy. Annual Review of Medicine 39: 405–423, 1988b
Collen D, Topol EJ, Tiefenbrunn AJ, Gold HK, Weisfeldt ML, et al. Coronary thrombolysis with recombinant human tissue-type plasminogen activator: a prospective, randomized, placebo-controlled trial. Circulation 70: 1012–1017, 1984b
Colucci M, Paramo JA, Collen D. Generation in plasma of a fast-acting inhibitor of plaminogen activator in response to endo-toxin stimulation. Journal of Clinical Investigation 75: 818–824, 1985
Colucci M, Stassen JM, Collen D. Influence of protein C activation on blood coagulation and fibrinolysis in squirrel monkeys. Journal of Clinical Investigation 74: 200–204, 1984b
Colucci M, Stassen JM, Salwa J, Collen D. Identification of plasminogen activator releasing activity in the neurohypophysis. British Journal of Haematology 58: 337–346, 1984a
Comp PC, Esmon CT. Generation of fibrinolytic activity by infusion of activated protein C into dogs. Journal of Clinical Investigation 68: 1221–1228, 1981
Cranston RE, Wolfson MA, Buchsbaum HW, Feinberg WM, Barreuther A. Plasminogen activator and cerebral infarction. An-nals of Internal Medicine 108: 766, 1988
Degen SFJ, Rajput B, Reich E. The human tissue plasminogen activator gene. Journal of Biological Chemistry 261: 6972–6985, 1986
del Zoppo GJ. Investigational use of tPA in acute stroke. Annals of Emergency Medicine 17: 1196–1201, 1988
Del Zoppo GJ, Copeland BR, Waltz TA, et al. The beneficial effect of intracarotid urokinase on acute stroke in a baboon model. Stroke 17: 638–643, 1986
De Wood MA, Spores J, Notske R, Lowell T, Mouser T, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. New England Journal of Medicine 303: 897–902, 1980
De Zwaan C, Bar FW, Janssen JHA, De Swart HB, Vermeer F, et al. Effects of thrombolytic therapy in unstable angina: clinical and angiographic results. Journal of the American College of Cardiology 12: 301–309, 1988
Edlund T, Ny T, Ranby M, Heden L-O, Palm G, et al. Isolation of cDNA sequences coding for a part of human tissue plasminogen activator. Proceedings of the National Academy of Sciences (USA) 80: 349–352, 1983
Emeis JJ, van den Hoogen CM, Jense D. Hepatic clearance of tissue-type plasminogen activator in rats. Thrombosis and Haemostasis 54: 661–664, 1985
Erickson LA, Ginsberg MH, Loskutoff DJ. Detection and partial characterization of an inhibitor of plasminogen activator in human platelets. Journal of Clinical Investigation 74: 1465–1472, 1984
Fearnley GR, Chakrabarti R, Hocking ED. Fibrinolytic effects of diguanides plus ethyloestrenol in occlusive vascular disease. Lancet 2: 1008–1011, 1967
Fisher R, Waller EK, Grossi G, Thompson D, Tizard R, et al. Isolation and characterization of the human tissue-type plasminogen activator structural gene including its 5′-flanking region. Journal of Biological Chemistry 260: 11223–11230, 1985
Flameng W, Van de Werf F, Vanhaecke J, Verstraete M, Collen D. Coronary thrombolysis and infarct size reduction after intravenous infusion of recombinant tissue-type plasminogen activator in nonhuman primates. Journal of Clinical Investigation 75: 84–90, 1985
Fong KLL, Crysler CS, Mico BA, Boyle KE, Kopia GA, et al. Dose-independent pharmacokinetics of recombinant tissue-type plasminogen activator in anesthetized dogs following intravenous infusion. Drug Metabolism and Disposition 16: 201–206, 1988
Fry ETA, Sobel BE. Lack of interference by heparin with thrombolysis or binding of tissue-type plaminogen activator to thrombi. Blood 71: 1347–1352, 1988
Fu KP, Lee S, Hum WT, Kalyan N, Rappaport R, et al. Disposition of a novel recombinant tissue plasminogen activator, Δ2-89 TPA, in mice. Thrombosis Research 50: 33–41, 1988
Fuchs HE, Berger H, Pizzo SV. Catabolism of human tissue plasminogen activator in mice. Blood 65: 539–544, 1985
Garabedian HD, Gold HK, Leinbach RC, Johns JA, Yasuda T, et al. Comparative properties of two clinical preparations of recombinant human tissue-type plasminogen activator in patients with acute myocardial infarction. Journal of the American College of Cardiology 9: 599–607, 1987
Gelehrter TD, Sznycer-Laszuk R. Thrombin induction of plasminogen activator-inhibitor in cultured human endothelial cells. Journal of Clinical Investigation 77: 165–169, 1986
Gelehrter TD, Sznycer-Laszuk R, Zeheb R, Cwikel BJ. Dexa-methasone inhibition of tissue-type plasminogen activator (t-PA) activity: paradoxical induction of both t-PA antigen and plasminogen activator inhibitor. Molecular Endocrinology 1: 97–101, 1987
GISSI (Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico). Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Lancet 1: 397–402, 1986
GISSI (Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico). Long-term effects of intravenous thrombolysis in acute myocardial infarction: final report of the GISSI study. Lancet 2: 871–874, 1987
Gold HK, Coller B, Yasuda T, Saito T, Fallon JT, et al. Rapid and sustained coronary artery recanalization with combined bolus injection of recombinant tissue-type plasminogen activator and monoclonal antiplatelet GPIIb/IIIa antibody in a canine preparation. Circulation 77: 670–677, 1988
Gold HK, Fallon JT, Yasuda T, Leinbach RC, Khaw BA, et al. Coronary thrombolysis with recombinant human tissue-type plasminogen activator. Circulation 70: 700–707, 1984
Gold HK, Johns JA, Leinbach RC, Yasuda T, Collen D. Thrombolytic therapy for unstable angina pectoris: rationale and results. Journal of the American College of Cardiology 10: 91B-95B, 1987b
Gold HK, Johns JA, Leinbach RC, Yasuda T, Grossbard E, et al. A randomized, blinded, placebo-controlled trial of recombinant human tissue-type plasminogen activator in unstable angina pectoris. Circulation 75: 1192–1199, 1987a
Gold HK, Leinbach RC, Garabedian HD, Yasuda T, Johns JA, et al. Acute coronary reocclusion after thrombolysis with recombinant human tissue-type plasminogen activator: prevention by a maintenance infusion. Circulation 73: 347–352, 1986a
Gold HK, Leinbach RC, Johns JA, Yasuda T, Garabedian HD, et al. Prevention of coronary reocclusion and reduction in late coronary stenosis by maintenance recombinant tissue-type plasminogen activator (rt-PA) infusion. Circulation 74: 11–368, 1986b
Goldhaber SZ, Kessler CM, Heit J, Markis J, Sharma GVRK, et al. Randomised controlled trial of recombinant tissue plasminogen activator versus urokinase in the treatment of acute pulmonary embolism. Lancet 2: 293–298, 1988
Goldhaber S, Loscalzo J. Urokinase versus tissue plasminogen activator in pulmonary embolism. Lancet 2: 915, 1988
Goldhaber SZ, Markis JE, Kessler CM, Meyerovitz MF, Kim D, et al. Perspective on treatment of acute pulmonary embolism with tissue plasminogen activator. Seminars in Thrombosis and Haemostasis 13: 171–177, 1987a
Goldhaber SZ, Meyerovitz MF, Markis JE, Kim D, Kessler CM, et al. Thrombolytic therapy of acute pulmonary embolism: current status and future potential. Journal of the American College of Cardiology 10: 96B–104B, 1987b
Goldhaber SZ, Vaughan DE, Markis JE, Selwyn AP, Meyerovitz MF, et al. Acute pulmonary embolism treated with tissue plasminogen activator. Lancet 2: 886–889, 1986
Golino P, Ashton JH, Glas-Greenwalt P, McNatt J, Buja LM, et al. Mediation of reocclusion by thromboxane A2 and serotonin after thrombolysis with tissue-type plasminogen activator in a canine preparation of coronary thrombosis. Circulation 77: 678–684, 1988
Graor RA, Risius B. Thrombolysis with recombinant human tissue-type plasminogen activator in patients with peripheral artery and bypass graft thrombosis. In Sobel et al. (Eds) Tissue plasminogen activator in thrombolytic therapy, pp. 171–204, Marcel Dekker Inc., New York, 1987
Graor RA, Risius B, Young JR, Denny K, Beven EG, et al. Peripheral artery and bypass graft thrombolysis with recombinant human tissue-type plasminogen activator. Journal of Vascular Surgery 3: 115–124, 1986
Guerci AD, Gerstenblith G, Brinker JA, Chandra NC, Gottlieb SO, et al. A randomized trial of intravenous tissue plasminogen activator for acute myocardial infarction with subsequent randomization to elective coronary angioplasty. New England Journal of Medicine 317: 1613–1618, 1987
Hajjar KA, Hamel NM, Harpel PC, Nachman RL. Binding of tissue plasminogen activator to cultured human endothelial cells. Journal of Clinical Investigation 80: 1712–1719, 1987
Hajjar KA, Harpel PC, Jaffe EA, Nachman RL. Binding of plasminogen to cultured human endothelial cells. Journal of Biological Chemistry 261: 11656–11662, 1986
Hamsten A, Blombäck M, Wiman B, Svensson J, Szamosi A, et al. Haemostatic function in myocardial infarction. British Heart Journal 55: 58–66, 1986
Hamsten A, De Faire U, Walldius G, Dahlén G, Szamosi A, et al. Plasminogen activator inhibitor in plasma; risk factor for recurrent myocardial infarction. Lancet 2: 3–7, 1987
Hamsten A, Wiman B, De Faire U, Blombäck M. Increased plasma levels of a rapid inhibitor of tissue plasminogen activator in young survivors of myocardial infarction. New England Journal of Medicine 313: 1557–1563, 1985
Hanss M, Collen D. Secretion of tissue-type plasminogen activator and plasminogen activator inhibitor by cultured human endothelial cells: modulation by thrombin, endotoxin and histamine. Journal of Laboratory and Clinical Medicine 109: 97–104, 1987
Harris R, Frade LG, Creighton LJ, Gascoine PS, Alexandroni MM, et al. Investigation by HPLC of the catabolism of recombinant tissue plasminogen activator in the rat. Thrombosis and Haemostasis 60: 107–112, 1988
Harris TJR, Patel T, Marston FAO, Little S, Emtage S, et al. Cloning of cDNA coding for human tissue-type plasminogen activator and its expression in Escherichia coli. Molecular Biology in Medicine 3: 279–292, 1986
Henze T, Boeer A, Tebbe U, Romatowski J. Lysis of basilar artery occlusion with tissue-plasminogen activator. Lancet 2: 1391, 1987
Hergrueter CA, Handren J, Kersh R, May Jr JW. Human recombinant tissue type plasminogen activator and its effect on microvascular thrombosis in the rabbit. Plastic and Reconstructive Surgery 81: 418–424, 1988
Higgins DL, Vehar GA. Interaction of one-chain and two-chain tissue plasminogen activator with intact and plasmin-degraded fibrin. Biochemistry 26: 7786–7791, 1987
Hillis LD, Borer J, Braunwald E, et al. High dose intravenous streptokinase for acute myocardial infarction: preliminary results of a multicenter trial. Journal of the American College of Cardiology 6: 957–962, 1985
Holvoet P, Boes J, Collen D. Measurement of free, one-chain tissue-type plasminogen activator in human plasma with anenzyme-linked immunosorbent assay based on an active sitespecific murine monoclonal antibody. Blood 69: 284–289, 1987
Holvoet P, Cleemput H, Collen D. Assay of human tissue-type plasminogen activator (t-PA) with an enzyme-linked immunosorbent assay (ELISA) based on three murine monoclonal antibodies to t-PA. Thrombosis and Haemostasis 54: 684–687, 1985
Holvoet P, Lijnen HR, Collen D. A monoclonal antibody preventing binding of tissue-type plasminogen activator to fibrin: useful to monitor fibrinogen breakdown during t-PA infusion. Blood 67: 1482–1487, 1986
Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator: role of fibrin. Journal of Biological Chemistry 257: 2912–2919, 1982
Ichinose A, Tamaki T, Aoki N. Factor XIII-mediated cross-linking of NH2-terminal peptide of alpha2-plasmin inhibitor to fibrin. FEBS Letters 153: 369–371, 1983
Isacson S, Nilsson IM. Defective fibrinolysis in blood and vein walls in recurrent “idiopathic” venous thrombosis. Acta Chirurgica Scandinavica 138: 313–319, 1972
ISAM Study Group. A prospective trial of intravenous streptokinase in acute myocardial infarction (ISAM). Mortality, morbidity, and infarct size at 21 days. New England Journal of Medicine 314: 1465–1471, 1986
ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 2: 349–360, 1988
Jaffe GJ, Green GDJ, McKay BS, Hartz A, Williams GA. Intravitreal clearance of tissue plasminogen activator in the rabbit. Archives of Ophthalmology 106: 969–972, 1988
Jang I, Vanhaecke J, De Geest H, Verstraete M, Collen D, et al. Coronary thrombolysis with recombinant tissue-type plasminogen activator: patency rate and regional wall motion after 3 months. Journal of the American College of Cardiology 8: 1455–1460, 1986
Johns JA, Gold HK, Leinbach RC, Yasuda T, Gimple LW, et al. Prevention of coronary artery reocclusion and reduction in late coronary artery stenosis after thrombolytic therapy in patients with acute myocardial infarction. A randomized study of maintenance infusion of recombinant human tissue-type plasminogen activator. Circulation 78: 546–556, 1988
Johnson RN, Olsen K, Hernandez E. Tissue plasminogen activator treatment of postoperative intraocular fibrin. Ophthalmology 95: 592–596, 1988
Jorgensen M, Bonnevie-Nielsen V. Increased concentration of the fast-acting plasminogen activator inhibitor in plasma associated with familial venous thrombosis. British Journal of Haematology 65: 175–180, 1987
JÖrnvall H, Pohl G, Bergsdorf N, Wallen P. Differential proteolysis and evidence for a residue exchange in tissue plasminogen activator suggest possible association between two types of protein microheterogeneity. FEBS Letters 156: 47–50, 1983
Juhan-Vague I, Moerman B, De Cock F, Aillaud MF, Collen D. Plasma levels of a specific inhibitor of tissue-type plasminogen activator (and urokinase) in normal and pathological conditions. Thrombosis Research 33: 523–530, 1984
Juhan-Vague I, Valadier J, Alessi MC, Aillaud MF, Ansaldi J, et al. Deficient t-PA release and elevated PA inhibitor levels in patients with spontaneous or recurrent deep venous thrombosis. Thrombosis and Haemostasis 57: 67–72, 1987
Kagitani H, Tagawa M, Hatanaka K, Ikari T, Saito A, et al. Expression in E. coli of finger-domain lacking tissue-type plasminogen activator with high fibrin affinity. FEBS Letters 189: 145–149, 1985
Kalyan NK, Lee SG, Wilhelm J, Fu KP, Hum WT, et al. Structure-function analysis with tissue-type plasminogen activator. Effect of deletion of NH2-terminal domains on its biochemical and biological properties. Journal of Biological Chemistry 263: 3971–3978, 1988
Kaufman R, Wasley LC, Spiliotes AJ, Gosseis SD, Latt SA, et al. Coamplification and coexpression of human tissue-type plasminogen activator and murine dihydrofolate reductase sequences in Chinese hamster ovary cells. Molecular and Cellular Biology 5: 1750–1759, 1985
Kennedy JW, Martin GV, Davis KB, et al. The Western Washington intravenous streptokinase in acute myocardial infarction randomized trial. Circulation 77: 345–352, 1988
Kennedy JW, Ritchie JL, Davis KB, et al. The Western Washington randomized trial of intravenous streptokinase in acute myocardial infarction: a 12-month follow-up. New England Journal of Medicine 312: 1073–1078,1985
Kennedy JW, Ritchie JL, Davis KB, Fritz JK. Western Washington randomized trial of intracoronary streptokinase in acute myocardial infarction. New England Journal of Medicine 309: 1477–1482, 1983
Kent RS, Batson AG, Littlejohn JK. Thrombolytic effect of tissue-type plasminogen activator. In Schroder R et al. (Eds) Controversies in thrombolysis, Current Medical Literature Ltd., London, in press, 1988
Kissel P, Chehrazi B, Seibert JA, Wagner FC. Digital angiographic quantification of blood flow dynamics in embolic stroke treated with tissue-type plasminogen activator. Journal of Neurosurgery 67: 399–405, 1987
Kluft C, Jie AFH, Lowe GDO, Blarney SL, Forbes CD. Association between postoperative hyper-response in t-PA inhibition and deep vein thrombosis. Thrombosis and Haemostasis 56: 107, 1986
Kooistra T, Van den Berg J, Töns A, Platenburg G, Rijken DC, et al. Butyrate stimulates tissue-type plasminogen-activator synthesis in cultured human endothelial cells. Biochemical Journal 247: 605–612, 1987
Korninger C, Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thrombosis and Haemostasis 46: 561–565, 1981
Korninger C, Lechner K, Niessner H, Gössinger H, Kundi M. Impaired fibrinolytic capacity predisposes for recurrence of venous thrombosis. Thrombosis and Haemostasis 52: 127–130, 1984
Korninger C, Matsuo O, Suy R, Stassen JM, Collen D. Thrombolysis with human extrinsic (tissue type) plasminogen activator in dogs with femoral vein thrombosis. Journal of Clinical Investigation 69: 573–580, 1982
Korninger C, Stassen JM, Collen D. Turnover of human extrinsic (tissue-type) plasminogen activator in rabbits. Thrombosis and Haemostasis 46: 658–661, 1981
Koudstaal PJ, Stibbe J, Vermeulen M. Fetal ischaemic brain oedema after early thrombosis with tissue plasminogen activator in acute stroke. British Medical Journal 297: 1571–1574, 1988
Kreutzer A, Brunner R, Schäfer H-J, Sickel W, Auel H, et al. Lysetherapie mit rt-PA bei Patienten mit Stamm-oder Zentral venenocclusion der Retina. Fortschritte der Ophthalmologie 85: 511–513, 1988
Kruithof EKO, Gudinchet A, Bachmann F. Plasminogen activator inhibitor 1 and plasminogen activator inhibitor 2 in various disease states. Thrombosis and Haemostasis 59, 7–12, 1988
Kruithof EKO, Ransijn A, Bachmann F. Inhibition of tissue plasminogen activator by human plasma. In Davidson et al. (Eds) Progress in fibrinolysis, Vol. 6, pp. 365–369, Churchill Livingstone, Edinburgh, 1983
Kruithof EKO, Schleuning WD, Bachmann F. Human tissue-type plasminogen activator. Production in continuous serum-free cell culture and rapid purification. Biochemical Journal 226: 631–636, 1985
Kuiper J, Otter M, Rijken DC, van Berkel TJC. Characterization of the interaction invivoof tissue-type plasminogen activator with liver cells. Journal of Biological Chemistry 263: 18220–18224, 1988
Lambrou FH, Snyder RW, Williams GA. Use of tissue plasminogen activator in experimental hyphema. Archives of Ophthalmology 105: 995–997, 1987
Larsen GR, Henson K, Blue Y. Variants of human tissue-type plasminogen activator. Fibrin binding, fibrinolytic, and fibrinogenolytic characterization of genetic variants lacking the fibronectin finger-like and/or the epidermal growth factor domains. Journal of Biological Chemistry 263: 1023–1029, 1988
Lau D, Kuzma G, Wei CM, Livingston DJ, Hsiung N. A modified human tissue plasminogen activator with extended half-life in vivo. Bio/technology 5: 953–958, 1987
Lefer AM, Mentley R, Sun J-Z. Potentiation of myocardial salvage by tissue type plasminogen activator in combination with a thromboxane synthetase inhibitor in ischemic cat myocardium. Circulation Research 63: 621–627, 1988
Lijnen HR, Marafino Jr BJ, Collen D. In vitro fibrinolytic activity of recombinant tissue-type plasminogen activator in the plasma of various primate species. Thrombosis and Haemostasis 52: 308–310, 1984
Liu CY, Wallen P. The binding of tissue plasminogen activator by fibrin. Abstract. Circulation 70: 365, 1984
Liu Y-X, Cajander SB, Ny T, Kristensen P, Hsueh AJW. Go-nadotropin regulation of tissue-type and urokinase-type plasminogen activators in rat granulosa and theca-interstitial cells during the periovulatory period. Molecular and Cellular Endocrinology 54: 221–229, 1987
Loscalzo J. Structural and kinetic comparison of recombinant human single-and two-chain tissue plasminogen activator. Journal of Clinical Investigation 82: 1391–1397, 1988
Loskutoff DJ, Linders M, Keijer J, Veerman H, van Heerik-huizen H, et al. Structure of the human plasminogen activator inhibitor 1 gene: nonrandom distribution of introns. Biochemistry 26: 3763–3768, 1987
Loskutoff DJ, van Mourik JA, Erickson LA, Lawrence D. Detection of an unusually stable fibrinolytic inhibitor produced by bovine endothelial cells. Proceedings of the National Academy of Sciences (USA) 80: 2956–2960, 1983
Lucore CL, Sobel BE. Interactions of tissue-type plasminogen activator with plasma inhibitors and their pharmacologic implications. Circulation 77: 660–669, 1988
Magnani B, Plasminogen Activator Italian Multicenter Study (PAIMS) Group. Comparison of intravenous recombinant single-chain human tissue-type plasminogen activator (rt-PA) with intravenous streptokinase in acute myocardial infarction. Journal of the American College of Cardiology 13: 19–26, 1989
Marder VJ, Francis CW. Thrombolytic therapy in acute transmural myocardial infarction. American Journal of Medicine 77: 921–928,1984
Marder VJ, Sherry S. Thrombolytic therapy: current status (first of two parts). New England Journal of Medicine 318: 1512–1520, 1988a
Marder VJ, Sherry S. Thrombolytic therapy: current status (second of two parts). New England Journal of Medicine 318: 1585–1595, 1988b
Matsuo O, Rijken DC, Collen D. Comparison of the relative fibrinogenolytic, fibrinolytic and thrombolytic properties of tissue plasminogen activator and urokinase in vitro. Thrombosis and Haemostasis 45: 225–229, 1981a
Matsuo O, Rijken DC, Collen D. Thrombolysis by human tissue plasminogen activator and urokinase in rabbits with experimental pulmonary embolus. Nature 291: 590–591, 1981b
Matsuo O, Okada K, Fukao H, Tanaka N, Ueshima S. Monoclonal antibody interferes with fibrin binding of t-PA. Thrombosis Research 51: 485–494, 1988
Mattsson C, Nyberg-Arrhenius V, Wallen P. Dissolution of thrombi by tissue plasminogen activator, urokinase and streptokinase in an artificial circulating system. Thrombosis Research 21: 535–545, 1981
Mattsson CH, Nilsson S, Häggroth L. Human extrinsic plasminogen activator: fibrinolytic properties and neutralization in vivo. Thrombosis Research 30: 91–100, 1983
McNeill AJ, Adgey AAJ. Thrombolytic therapy for myocardial infarction. Lancet 1: 938–939, 1988
McNeill AJ, Shannon JS, Cunningham SR, Flannery DJ, Campbell NPS, et al. A randomised dose ranging study of recombinant tissue plasminogen activator in acute myocardial infarction. British Medical Journal 296: 1768–1771, 1988
Mehta J, Mehta P, Lawson D, Saldeen T. Plasma tissue plasminogen activator inhibitor levels in coronary artery disease: correlation with age and serum triglyceride concentrations. Journal of the American College of Cardiology 9: 263–268, 1987
Mellbring G, Dahlgren S, Reiz S, Wiman B. Fibrinolytic activity in plasma and deep vein thrombosis after major abdominal surgery. Thrombosis Research 32: 575–584, 1983
Mellbring G, Dahlgren S, Wiman B. Plasma fibrinolytic activity in patients undergoing major abdominal surgery. Acta Chirurgica Scandinavica 151: 109–114, 1985a
Mellbring G, Dahlgren S, Wiman B, Sunneg/oardh O. Relationship between preoperative status of the fibrinolytic system and occurrence of deep vein thrombosis after major abdominal surgery. Thrombosis Research 39: 157–163, 1985b
Miles LA, Plow EF. Binding and activation of plasminogen on the platelet surface. Journal of Biological Chemistry 260: 4303–4311, 1985
Mohr JP, Caplan LR, Melski JW, Goldstein RJ, Duncan GW, et al. The Harvard Cooperative Stroke Registry: a prospective registry. Neurology 28: 754–762, 1978
Mueller HS, Roa AK, Forman SA. Thrombolysis in Myocardial Infarction (TIMI): comparative studies of coronary reperfusion and systemic fibrinogenolysis with two forms of recombinant tissue-type plasminogen activator. Journal of the American College of Cardiology 10: 479–490, 1987
National Heart Foundation of Australia Coronary Thrombolysis Group. Coronary thrombolysis and myocardial salvage by tissue plasminogen activator given up to 4 hours after onset of myocardial infarction. Lancet 1: 203–208, 1988
Neuhaus KL, Tebbe U, Gottwik M, et al. Intravenous recombinant tissue plasminogen activator (rt-PA) and urokinase in acute myocardial infarction: results of the German Activator Urokinase Study (GAUS). Journal of the American College of Cardiology 12: 581–587, 1988
Neuhaus KL, Tebbe U, Sauer G, Rahlf G, Kreuzer H, et al. Hoch-dosierte intravenöse Kurzinfusion von Streptokinase beim acuten Myocardinfarkt. In Trubestein & Etzel (Eds) Fibrinolytische Therapie, pp. 425–430, FK Schattauer Verlag, Stuttgart, 1983
Nicklas JM, Topol EJ, Kander N, Walton JA, Gorman L, et al. Randomized, double-blind, placebo-controlled trial of rt-PA in unstable angina. Circulation 76: IV–305, 1987
Nilsson IM, Hedner U, Isacson S. Phenformin and ethyloestrenol in recurrent venous thrombosis. Acta Medica Scandinavica 198: 107–113, 1975
Nilsson IM, Ljungnér H, Tengborn L. Two different mechanisms in patients with venous thrombosis and defective fibrinolysis: low concentration of plasminogen activator or increased concentration of plasminogen activator inhibitor. British Medical Journal 290: 1453–1455, 1985a
Nilsson S, Einarsson M, Ekvärn S, Häggroth L, Mattsson CH. Turnover of tissue plasminogen activator in normal and hepatectomized rabbits. Thrombosis Research 39: 511–521, 1985b
Norrman B, Wallen P, Ranby M. Fibrinolysis mediated by tissue plasminogen activator. Disclosure of a kinetic transition. European Journal of Biochemistry 149: 193–200, 1985
Ny T, Elgh F, Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proceedings of the National Academy of Sciences (USA) 81: 5355–5359, 1984
Ny T, Liu Y-X, Ohlsson M, Jones PBC, Hsueh AJW. Regulation of tissue-type plasminogen activator activity and messenger RNA levels by gonadotropin-releasing hormone in cultured rat granulosa cells and cumulus-oocyte complexes. Journal of Biological Chemistry 262: 11790–11793, 1987
O’Connell ML, Canipari R, Strickland S. Hormonal regulation of tissue plasminogen activator secretion and mRNA levels in rat granulosa cells. Journal of Biological Chemistry 262: 2339–2344, 1987
O’Rourke M, Baron D, Keogh A, Kelly R, Nelson G, et al. Limitation of myocardial infarction by early infusion of recombinant tissue-type plasminogen activator. Circulation 77: 1311–1315, 1988
Pannekoek H, Veerman H, Lambers H, Diergaarde P, Verweij CL, et al. Endothelial plasminogen activator inhibitor (PAI): a new member of the serpin gene family. EMBO Journal 5: 2539–2544,1986
Papadopoulous SM, Chandler WF, Salamat MS, Topol EJ, Sackellares JC. Recombinant human tissue-type plasminogen activator therapy in acute thromboembolic stroke. Journal of Neurosurgery 67: 394–398, 1987
Páramo JA, Alfaro MJ, Rocha E. Postoperative changes in the plasmatic levels of tissue-type plasminogen activator and its fast-acting inhibitors: relationship to deep vein thrombosis and influence of prophylaxis. Thrombosis and Haemostasis 54: 713–716, 1985a
Páramo JA, Colucci M, Collen D, Van de Werf F. Plasminogen activator inhibitor in the blood of patients with coronary artery disease. British Medical Journal 291: 573–574, 1985b
Passamani E, Hodges M, Herman M, Grose R, Chaitman M, et al. The thrombolysis in Myocardial Infarction (TIMI) Phase II Pilot Study: tissue plasminogen activator followed by percutaneous transluminal coronary angioplasty. Journal of the American College of Cardiology 10: 51B–64B, 1987
Penar PL, Gréer CA. The effect of intravenous tissue-type plasminogen activator in a rat model of embolic cerebral ischemia. Yale Journal of Biology and Medicine 60: 233–243, 1987
Pennica D, Holmes WE, Kohr WJ, Harkins RN, Vehar GA, et al. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli Nature 301: 214–221, 1983
Phillips DA, Fischer M, Smith TW, Davis MA. The safety and angiographic efficacy of tissue plaminogen activator in a cerebral embolization model. Annals of Neurology 23: 391–394, 1988
Rajput B, Degen SF, Reich E, Walker EK, Axelrod J, et al. Chromosomal locations of human tissue plasminogen activator and urokinase genes. Science 2: 672–674, 1985
Rakoczi I, Chamone D, Collen D, Verstraete M. Prediction of postoperative leg-vein thrombosis in gynaecological patients. Lancet 1: 509–510, 1978
Ranby M. Studies on the kinetics of plasminogen activation by tissue plasminogen activator. Biochimica et Biophysica Acta 704: 461–469, 1982
Rao KA, Pratt C, Berke A, Jaffe A, Ockene I, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial — Phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. Journal of the American College of Cardiology 11: 1–11, 1988
Raynaud P, Desveaux B. Réocclusion après traitement par l’Actilyse. Archives des Maladies du Coeur et des Vaisseaux 81 (Suppl. 1): 25–32, 1988
Reddy VB, Garramone AJ, Sasak H, Wei CM, Watkins P, et al. Expression of human uterine tissue-type plasminogen activator in mouse cells using BPV vectors. Journal of Molecular Biology 6: 461–472, 1987
Rentrop KP, Feit F, Blanke H, et al. Effects of intracoronary streptokinase and intracoronary nitroglycerin infusion on coronary angiographic patterns and mortality in patients with acute myocardial infarction. New England Journal of Medicine 311: 1457–1463, 1984
Rijken DC, Collen D. Purification and characterization of the plasminogen activator secreted by human melanoma cells in culture. Journal of Biological Chemistry 256: 7035–7041, 1981
Rijken DC, Emeis JJ. Clearance of the heavy and light polypeptide chains of human tissue-type plasminogen activator in rats. Biochemical Journal 238: 643–646, 1986
Rijken DC, Hoylaerts M, Collen D. Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. Journal of Biological Chemistry 257: 2920–2925, 1982
Rijken DC, Juhan-Vague I, Collen D. Complexes between tissue-type plasminogen activator and proteinase inhibitors in human plasma, identified with an immunoradiometric assay. Journal of Laboratory and Clinical Medicine 101: 285–294, 1983b
Rijken DC, Juhan-Vague I, De Cock F, Collen D. Measurement of human tissue-type plasminogen activator by a two-site immunoradiometric assay. Journal of Laboratory and Clinical Medicine 101: 274–284, 1983a
Rijken DC, Wijngaards G, Welbergen J. Relationship between tissue plasminogen activator and the activators in blood and vascular wall. Thrombosis Research 18: 815–830, 1980
Rijken DC, Wijngaards G, Zaal-de Jong M, Welbergen J. Purification and partial characterization of plasminogen activator from human uterine tissue. Biochimica et Biophysica Acta 580: 140–153, 1979
Risius B, Graor RA, Geisinger MA, Zelch MG, Lucas FV, et al. Thrombolytic therapy with recombinant human tissue-type plasminogen activator: a comparison of two doses. Radiology 164: 465–468, 1987
Robin P, Gruel Y, Lang M, Lagarrigue F, Scotto JM. Complete thrombolysis of mesenteric vein occlusion with recombinant tissue-type plasminogen activator. Lancet 1: 1391, 1988
Rogers WJ, Mantte JA, Hood WP, et al. Prospective randomized trial of intravenous and intracoronary streptokinase in acute myocardial infarction. Circulation 68: 1051–1061, 1983
Ryan TJ. Angioplasty in myocardial infarction. Is the balloon leaking? New England Journal of Medicine 317: 624–626, 1987
Sakata Y, Aoki N. Significance of cross-linking of alpha2-plasmin inhibitor to fibrin in inhibition of fibrinolysis and in hemostasis. Journal of Clinical Investigation 69: 536–542, 1982
Sambrook J, Hanahan D, Rodgers L, Gething M-J. Expression of human tissue-type plasminogen activator from lytic viral vectors and in established cell lines. Molecular Biology in Medicine 3: 459–481, 1986
Sampol J, Mercier C, Houel F, David G, Daver J. Studies of the thrombolytic action of a tissue plasminogen activator in dogs. In Davidson et al. (Eds) Progress in fibrinolysis, Vol. 6, pp. 463–466, Churchill Livingstone, Edinburgh, 1983
Schaer BH, Ross AM, Wasserman AG. Reinfarction, recurrent angina, and reocclusion after thrombolytic therapy. Circulation 76 (Suppl. II): 57–62, 1987
Schröder R, Biamino G, von Leitner ER, et al. Intravenous short-term infusion of streptokinase in acute myocardial infarction. Circulation 67: 536–548, 1983
Seifried E, Tanswell P. Comparison of specific antibody, D-Phe-Pro-Arg-Ch2C1 and aprotinin for prevention of in vitro effects of recombinant tissue-type plasminogen activator on haemostatis parameters. Thrombosis and Haemostasis 58: 921–926, 1987
Seifried E, Tanswell P, Rijken DC, Barrett-Bergshoeff MM, Su CAPF, et al. Pharmacokinetics of antigen and activity of recombinant tissue-type plasminogen activator after infusion in healthy volunteers. Arzneimittel-Forschung 38: 418–422, 1988
Sheehan FH, Braunwald E, Canner P, Dodge HT, Gore J, et al. The effect of intravenous thrombolytic therapy on left ventricular function: a report on tissue-type plasminogen activator and streptokinase from the Thrombolysis in Myocardial Infarction (TIMI; Phase I) Trial. Circulation 75: 817–829, 1987
Sherman CT, Litvack F, Grundfest W, Lee M, Hickey A, et al. Coronary angioscopy in patients with unstable angina pectoris. New England Journal of Medicine 315: 913–919, 1986
Simoons ML, Arnold AER, Betriu A, de Bono DP, Col J, et al. Thrombolysis with tissue plasminogen activator in acute myocardial infarction: no additional benefit from immediate percutaneous coronary angioplasty. Lancet 1: 197–202, 1988
Simoons ML, Verstraete M. Thrombolytic therapy. Correspondence. New England Journal of Medicine 319: 1545, 1988
Slivka A, Pulsinelli W. Hemorrhagic complications of thrombolytic therapy in experimental stroke. Stroke 18: 1148–1156, 1987
Sloan MA. Thrombolysis and stroke. Past and future. Archives of Neurology 44: 748–768, 1987
Snyder RW, Lambrou FH, Williams GA. Intraocular fibrinolysis with recombinant human tissue plasminogen activator. Archives of Ophthalmology 105: 1277–1280, 1987
Sobel BE, Fields LE, Robison AK, Fox KAA, Sarnoff SJ. Coronary thrombolysis with facilitated absorption of intramuscularly injected tissue-type plasminogen activator. Proceedings of the National Academy of Sciences (USA) 82: 4258–4262, 1985
Sobel BE, Saffitz JE, Fields LE, Myears DW, Sarnoff SJ, et al. Intramuscular administration of human tissue-type plasminogen activator in rabbits and dogs and its implications for coronary thrombolysis. Circulation 75: 1261–1272, 1987
Soeda S, Kakiki M, Shimeno H, Nagamatsu A. Some properties of tissue-type plasminogen activator reconstituted onto phospholipid and/or glycolipid vesicles. Biochemical and Biophysical Research Communications 146: 94–100, 1987
Spann JF, Sherry S, Carabello BA, et al. Coronary thrombolysis by intravenous streptokinase in acute myocardial infarction: acute and follow-up studies. American Journal of Cardiology 53: 655–661, 1984
Sprengers ED, Kluft C. Plasminogen activator inhibitors. Blood 69: 381–387, 1987
Stack RS, O’Connor CM, Mark DB, et al. Coronary perfusion during acute myocardial infarction with a combined therapy of coronary angioplasty and high-dose intravenous streptokinase. Circulation 77: 151–161, 1988
Stalder M, Hauert J, Kruithof EKO, Bachmann F. Release of vascular plasminogen activator (v-PA) after venous stasis: electrophoretic-zymographic analysis of free and complexed v-PA. British Journal of Haematology 61: 169–176, 1985
Stassen JM, Juhan-Vague I, Alessi MC, De Cock F, Collen D. Potentiation by heparin fragments of thrombolysis induced with human tissue-type plasminogen activator or human single chain urokinase-type plasminogen activator. Thrombolysis and Haemostasis 58: 947–950, 1987
Steiner TJ. Fatal ischaemic brain oedema after tissue plasminogen activator Correspondence. British Medical Journal 298: 382, 1989
Stewart A, Mayne EE. Rapid resolution of subclavian vein thrombosis by tissue plasminogen activator. Lancet 1: 890, 1988
Stricker RB, Wong D, Tak Shiu D, Reyes PT, Shuman MA. Activation of plasminogen by tissue plasminogen activator on normal and thrombasthenic platelets: effects on surface proteins and platelet aggregation. Blood 68: 275–280, 1986
Stump DC, Topol EJ, Califf R, Chen AJ, Hopkins A, et al. Results of coagulation-fibrinolysis analyses in 386 patients with acute myocardial infarction treated with recombinant tissue-type plasminogen activator (rt-PA) (TAMI-trial). Abstract no. 947. Thrombosis and Haemostasis 58: 259, 1987
Suenson E, Lützen O, Thorsen S. Initial plasmin-degradation of fibrin as the basis of a positive feed-back mechanism in fibrinolysis. European Journal of Biochemistry 140: 513–522, 1984
Takada A, Hou P, Mori T, Takada Y. Changes in various parameters of fibrinolysis in persons infused with tissue plasminogen activator: special reference to plasminogen activator inhibitor. Thrombosis Research, in press, 1988b
Takada A, Watahiki Y, Takada Y. Release of N-terminal peptides from Glu-plasminogen by plasmin in the presence of fibrin. Thrombosis Research 41: 819–827, 1986
Takada A, Mochizuki K, Takada Y. Influence of SK-potentiator and fibrinogen degradation products on the activation of human plasminogen by streptokinase. Thrombosis Research 22: 623–631, 1981
Takada A, Takada Y. Activation of Glu-plasminogen by urokinase in the presence of fibrinogen, fibrin, and SK-potentiator. Thrombosis Research 22: 497–501, 1981
Takada Y, Takada A. Kinetic analyses of potentiation of plasminogen activation by streptokinase in the presence of fibrin or its degradation products. Haemostasis 17: 1–7, 1987
Takada A, Sugawara Y, Takada Y. Comparison of kinetic parameters of the activation of Glu-plasminogen by tissue plasminogen activator obtained from various sources. Haemostasis 18: 117–125, 1988a
Takada A, Takada Y, Sugawara Y. Effects of fibrinogen and fibrin on the activation of Glu-and Lys-plasminogen by urokinase. Thrombosis Research 33: 561–569, 1984
Themen ML. Streptokinase or TPA for acute myocardial infarction: is one agent superior? Cardiovascular Reviews and Reports 9: 55–64, 1988
Thorsen S, Philips M. Isolation of tissue-type plasminogen activator-inhibitor complexes from human plasma: evidence for a rapid plasminogen activator inhibitor. Biochimica et Biophysica Acta 802: 111 -118, 1984
TIMI Research Group. The comparison of immediate versusdelayed catheterization and angioplasty following thrombolytic therapy for acute myocardial infarction (TIMI IIa). Journal of the American Medical Association 260: 2849–2858 1988
TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) Trial: Phase I findings. New England Journal of Medicine 312: 932–936, 1985
TIMI Study Group. Comparison of invasive and conservative strategies after treatment with intravenous tissue plasminogen activator in acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) phase II trial. New England Journal of Medicine 320: 618–627, 1989
Tomaru T, Uchida Y, Nakamura F, Sonoki H, Tsukamoto M, et al. Enhancement of arterial thrombolysis with native tissue type plasminogen activator by pretreatment with heparin or batroxobin: an angioscopic study. American Heart Journal 117: 275–281, 1989
Topol EJ, Bates ER, Walton JA, et al. Community hospital administration of intravenous tissue plasminogen activator in acute myocardial infarction: improved timing, thrombolytic efficacy and ventricular function. Journal of the American College of Cardiology 10: 1173–1177, 1987a
Topol EJ, Califf RM, George BS, Kereiakis DJ, Abbottsmith CW, et al. A randomized trial of immediate versus delayed elective angioplasty after intravenous tissue plasminogen activator in acute myocardial infarction. New England Journal of Medicine 317: 581–588, 1987c
Topol EJ, Califf RM, George BS, Kereiakis DJ, Rothbaum D, et al. Coronary arterial thrombolysis with combined infusion of recombinant tissue-type plasminogen activator and urokinase in patients with acute myocardial infarction. Circulation 77: 1100–1107, 1988a
Topol EJ, Eha JE, Brin KB, Shapiro EP, Weiss JL, et al. Applicability of percutaneous transluminal coronary angioplasty to patients with recombinant tissue plasminogen activator mediated thrombolysis. Catheterization and Cardiovascular Diagnosis 11: 337–348, 1985
Topol EJ, George BS, Kereiakes DJ, Candela RJ, Abbottsmith CW, et al. Comparison of two dose regimens of intravenous tissue plasminogen activator for acute myocardial infarction. American Journal of Cardiology 61: 723–728, 1988c
Topol EJ, Morris DC, Smalling RW, Schumacher RR, Taylor CR, et al. A multicenter, randomized, placebo-controlled trial of a new form of intravenous recombinant tissue-type plasminogen activator (Activase) in acute myocardial infarction. Journal of the American College of Cardiology 9: 1205–1213, 1987b
Topol EJ, Nicklas JM, Kander NH, Walton JA, Ellis SG, et al. Coronary revascularization after intravenous tissue plasminogen activator for unstable angina pectoris: results of a randomized, double-blind, placebo-controlled trial. American Journal of Cardiology 62: 368–371, 1988d
Topol EJ, O’Neill WW, Langburd AB, Walton JA, Bourdillon PDV, et al. A randomized, placebo-controlled trial of intravenous recombinant tissue-type plasminogen activator and emergency coronary angioplasty in patients with acute myocardial infarction. Circulation 75: 420–428, 1987d
Tran-Chang C, Wyss P, Kruithof EKO, Hauert J, Bachmann F. Tissue-type plasminogen activator increases the binding of plasminogen to clots. Abstract. Haemostasis 14: 17, 1984
Tripathi RC, Park JK, Tripathi BJ, Millard CB. Tissue plasminogen activator in human aqueous humor and its possible therapeutic significance. American Journal of Ophthalmology 106: 719–722, 1988
Turpie AGG. Thrombolytic therapy in venous thromboembolism. In Sobel et al. (Eds) Tissue plasminogen activator in thrombolytic therapy, pp. 131–146, Marcel Dekker Inc., New York, 1987
Upshall A, Kumar AA, Bailey MC, Parker MD, Favreau MA, et al. Secretion of active human tissue plasminogen activator from the filamentous fungus Aspergillus-nidulans. Bio/Technology 5: 1301–1304, 1987
Urano T, de Serrano VS, Gaffney PJ, Castellino FJ. Effectors of the activation of human [Glu’] plasminogen by human tissue plasminogen activator. Biochemistry 27: 6522–6528, 1988
Van de Werf. Discrepancies between the effects of coronary reperfusion on left ventricular function and survival. Lancet, in press, 1989
Van de Werf F, Arnold AER, the European Cooperative Study Group for Recombinant Tissue-Type Plasminogen Activator. Intravenous tissue plasminogen activator and size of infarct, left ventricular function, and survival in acute myocardial infarction. British Medical Journal 297: 1374–1379, 1988
Van de Werf F, Bergmann SR, Fox KAA, De Geest H, Hoyng CF, et al. Coronary thrombolysis with intravenously administered human tissue-type plasminogen activator produced by recombinant DNA technology. Circulation 69: 605–610, 1984b
Van de Werf F, Ludbrook PA, Bergmann SR, Tiefenbrunn AJ, Fox KAA, et al. Coronary thrombolysis with tissue-type plasminogen activator in patients with evolving myocardial infarction. New England Journal of Medicine 310: 609–613, 1984a
Van de Werf F, Vanhaecke J, Jang IK, Flameng W, Collen D, et al. Reduction in infarct size and enhanced recovery of systolic function after coronary thrombolysis with tissue-type plasminogen activator combined with β-adrenergic blockade with metoprolol. Circulation 75: 830–836, 1987
van Hinsbergh VWM, Bertina RM, van Wijngaarden A, van Tilburg NH, Emeis JJ, et al. Activated protein C decreases plasminogen activator-inhibitor activity in endothelial cell-conditioned medium. Blood 65: 444–451, 1985
van Zonneveld AJ, Chang GTG, van den Berg J, Kooistra T, Verheijen JH, et al. Quantification of tissue-type plasminogen activator (t-PA) mRNA in human endothelial-cell cultures by hybridization with a t-PA cDNA probe. Biochemical Journal 235: 385–390, 1986b
van Zonneveld AJ, Veerman H, Pannekoek H. On the interaction of the finger and kringle-2 domain of tissue-type plasminogen activator with fibrin. Inhibition of kringle-2 binding to fibrin by E-amino caproic acid. Journal of Biological Chemistry 261: 14214–14218, 1986a
van Zonneveld AJ, Veerman H, Pannekoek H. Autonomous functions of structural domains of human tissue-type plasminogen activator. Proceedings of the National Academy of Science (USA) 83: 4670–4674, 1986c
Vaughan DE, Plavin SR, Schafer AI, Loscalzö J. Prostaglandin E1 markedly accelerates thrombolysis by tissue plasminogen activator. Blood 73: 1213–1217, 1989
Vehar GA, Spellman MW, Keyt BA, Ferguson CK, Keck RG, et al. Characterization studies of human tissue-type plasminogen activator produced by recombinant DNA technology. Cold Spring Harbor Symposia on Quantitative Biology, Vol. LI, pp. 551–562, Cold Spring Harbor Laboratory, 1986
Verheijen JH, Caspers MPM, Chang GTG, de Munk GAW, Pauwels PH, et al. Involvement of finger domain and kringle 2 domain of tissue-type plasminogen activator in fibrin binding and stimulation of activity by fibrin. EMBO Journal 5: 3525–3530, 1986a
Verheijen JH, Visse R, Wijnen JTh, Chang GTG, Kluft C, et al. Assignment of the human tissue-type plasminogen activator gene (PLAT) to chromosome 8. Human Genetics 72: 153–156, 1986b
Verheugt FWA, ten Cate JW, Sturk A, Imandt L, VerhÖrst PMJ, et al. Tissue plasminogen activator activity and inhibition in acute myocardial infarction and angiographically normal coronary arteries. American Journal of Cardiology 59: 1075–1079, 1987
Verstraete M, Arnold AER, Brower RW, Collen D, de Bono DP, et al. Acute coronary thrombolysis with recombinant human tissue-type plasminogen activator: initial patency and influence of maintained infusion on reocclusion rate. American Journal of Cardiology 60: 231–237, 1987
Verstraete M, Bernard R, Bory M, Brower RW, Collen D, et al. Randomised trial of intravenous recombinant tissue-type plasminogen activator versus intravenous streptokinase in acute myocardial infarction. Lancet 1: 842–847, 1985b
Verstraete M, Bleifeld W, Brower RW, Charbonnier B, Collen D, et al. Double-blind randomised trial of intravenous tissue-type plasminogen activator versus placebo in acute myocardial infarction. Lancet 2: 965–969, 1985c
Verstraete M, Bounameaux H, De Cock F, Van de Werf F, Collen D. Pharmacokinetics and systemic fibrinogenolytic effects of recombinant human tissue-type plasminogen activator (rt-PA) in man. Journal of Pharmacology and Experimental Therapeutics 235: 506–512, 1985a
Verstraete M, Collen D. Thrombolytic properties of t-PA in experimental venous occlusion and in patients with venous thrombosis. In Collen et al. (Eds) Thrombolysis: biological and therapeutic properties of new thrombolytic agents, pp. 49–60, Churchill Livingstone, Edinburgh, 1985
Verstraete M, Hess H, Mahler F, Mietaschk A, Roth F-J, et al. Femoro-popliteal artery thrombolysis with intra-arterial infusion of recombinant tissue-type plasminogen activator: report of a pilot trial. European Journal of Vascular Surgery 2: 155–159, 1988b
Verstraete M, Miller GAH, Bounameaux H, Charbonnier B, Colle JP, et al. Intravenous and intrapulmonary recombinant tissue-type plasminogen activator in the treatment of acute massive pulmonary embolism. Circulation 77: 353–360, 1988a
Verstraete M, Su CAPF, Tanswell P, Feuerer W, Collen D. Pharmacokinetics and effects on fibrinolytic and coagulation parameters of two doses of recombinant tissue-type plasminogen activator in healthy volunteers. Thrombosis and Haemostasis 56: 1–5, 1986
Vine AK, Maguire PT, Martonyi C, Kincaid MC. Recombinant tissue plasminogen activator to lyse experimentally induced retinal arterial thrombi. American Journal of Ophthalmology 105: 266–270, 1988
Voskuilen M, Vermond A, Veeneman GH, f Boom JH, Klasen EA, et al. Fibrinogen lysine residue A alpha 157 plays a crucial role in the fibrin-induced acceleration of plasminogen activation catalyzed by tissue-type plasminogen activator. Journal of Biological Chemistry 262: 5944–5946, 1987
Wallen P, Bergsdorf N, Ranby M. Purification and identification of two structural variants of porcine tissue plasminogen activator by affinity adsorption on fibrin. Biochimica et Biophysica Acta 719: 318–328, 1982
Wallen P, Pohl G, Bergsdorf N, Ranby M, Ny T, et al. Purification and characterisation of a melanoma cell plasminogen activator. European Journal of Biochemistry 132: 681–686, 1983
Walsh DG, Kaplan LR, Burney RE, Topol EJ, O’Neill WW, et al. Use of tissue plasminogen activator in the emergency department for acute myocardial infarction. Annals of Emergency Medicine 16: 243–247, 1987
Weimar W, Stibbe J, Van Seyen AJ, Billiau A, De Somer P, et al. Specific lysis of an iliofemoral thrombus by administration of extrinsic (tissue-type) plasminogen activator. Lancet 2: 1018–1020, 1981
White HD. Comparison of tissue plasminogen activator and streptokinase in the management of acute myocardial infarction. Chest, in press, 1989
White HD, Rivers JT, Maslowski AH, Ormiston JA, Takayama M, et al. Effect of intravenous streptokinase as compared with that of tissue plasminogen activator on left ventricular function after first myocardial infarction. New England Journal of Medicine 320: 817–821, 1989
White HD, Norris RM, Brown MA. The effects of intravenous streptokinase on left ventricular function and early survival after acute myocardial infarction. New England Journal of Medicine 317: 850–855, 1987
Wilcox RG, von der Lippe G, Olsson CG, Jenssen G, Skene AM, et al. Trial of tissue plasminogen activator for mortality reduction in acute myocardial infarction. Anglo-Scandinavian Study of Early Thrombolysis (ASSET). Lancet 2: 525–530, 1988
Williams DO, Borer J, Braunwald E, Chesebro JH, Coken LS, et al. Intravenous recombinant tissue-type plasminogen activator in patients with acute myocardial infarction: a report from the NHLBI thrombolysis in myocardial infarction trial. Circulation 73: 338–346, 1986
Williams GA, Lambrou FH, Jaffe GA, Snyder RW, Green GDJ, et al. Treatment of postvitrectomy fibrin formation with intraocular tissue plasminogen activator. Archives of Ophthalmology 106: 1055–1058, 1988
Wiman B, Boman L, Collen D. On the kinetics of the reaction between human antiplasmin and a low-molecular-weight form of plasmin. European Journal of Biochemistry 87: 143–146, 1978
Wiman B, Chmielewska J. A novel fast inhibitor to tissue plasminogen activator in plasma, which may be of great patho-physiological significance. Scandinavian Journal of Clinical and Laboratory Investigation 45 (Suppl. 177): 43–47, 1985
Wiman B, Chmielewska J, Ranby M. Inactivation of tissue plasminogen activator in plasma. Demonstration of a complex with a new rapid inhibitor. Journal of Biological Chemistry 259: 3644–3647, 1984
Wiman B, Collen D. On the kinetics of the reaction between human antiplasmin and plasmin. European Journal of Biochemistry 84: 573–578, 1978
Wiman B, Lijnen HR, Collen D. On the specific interaction between the lysine-binding sites in plasmin and complementary sites in α2-antiplasmin and in fibrinogen. Biochimica et Biophysica Acta 579: 142–154, 1979
Wiman B, Wallen P. The specific interaction between plasminogen and fibrin. A physiological role of the lysine binding site in plasminogen. Thrombosis Research 10: 213–222, 1977
Yang-Feng TL, Opdenakker G, Volckaert G, Francke U. Human tissue-type plasminogen activator gene located near chromo-somal breakpoint in myeloproliferative disorder. American Journal of Human Genetics 39: 79–87, 1986
Yusuf S, Collins R, Peto R, et al. Intravenous and intracoronary fibrinolytic therapy in acute myocardial infarction: overview of results on mortality, reinfarction and side-effects from 33 randomised controlled trials. European Heart Journal 6: 556–585, 1985
Zamarron C, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by natural and recombinant tissue-type plasminogen activator. Journal of Biological Chemistry 259: 2080–2083, 1984
Zimmerman R, Horn A, Harenberg J, Diehm C, Müller-Bühl U, et al. Thrombolysetherapie der tiefen venösen Thrombose mit rt-PA. Klinische Wochenschrift 66 (Suppl. XII): 137–142, 1988
Zivin JA, Fisher M, De Girolami V, Hemenway CC, Stashak JA. Tissue plasminogen activator reduces neurological damage after cerebral embolism. Science 230: 1289–1292, 1985
Zivin JA, Hemenway CC, De Girolami V. Delayed therapy of embolic stroke with tissue plasminogen activator. Annals of Neurology 20: 154, 1986
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: P. Desnoyers, Laboratoire Central d’Hématologie, Hôtel-Dieu, Paris, France; DJ. Kereiakes, Cincinnati, Ohio, USA; V.J. Marder, University of Rochester Medical Center, Rochester, New York, USA; M. Nidorf, Sir Charles Gairdner Hospital, Nedlands, Western Australia, Australia; D.C. Rijken,Gaubius Institute, Leiden, The Netherlands; E. Seifried, Medizinische Universitätsklinik und Poliklinik, Universität Ulm, Ulm, West Germany; B.E. Sobel, Department of Internal Medicine, Cardiovascular Division, Washington School of Medicine, St Louis, Missouri, USA; A. Takada, Department of Physiology, School of Medicine, Hamamatsu University, Hamamatsu, Japan; P. Thompson, Sir Charles Gairdner Hospital, Nedlands, Western Australia, Australia; E.J. Topol, Division of Cardiology, University of Michigan Medical Center, Ann Arbor, Michigan, USA; S. Verheught, Department of Cardiology, Free University Hospital, Amsterdam, The Netherlands; M. Verstraete, Centre for Thrombosis and Vascular Research, University of Leuven, Leuven, Belgium; Harvey D. White, Cardiology Department, Greenlane Hospital, Auckland, New Zealand.
Rights and permissions
About this article
Cite this article
Collen, D., Lijnen, H.R., Todd, P.A. et al. Tissue-Type Plasminogen Activator. Drugs 38, 346–388 (1989). https://doi.org/10.2165/00003495-198938030-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-198938030-00003