Abstract
Objective: To evaluate the effect of recombinant human erythropoietin (rHuEPO) on the reticulocyte production rate and age distribution in healthy subjects.
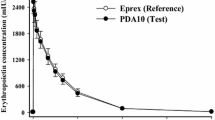
Methods: Extensive pharmacokinetic and pharmacodynamic data collected from 88 subjects who received a single subcutaneous dose of rHuEPO (dose range 20–160 kIU) were analysed. Four nonlinear mixed-effects models were evaluated to describe the time course of the percentage of reticulocytes and their age distribution in relation to rHuEPO pharmacokinetics. Model A accounted for stimulation of the production of progenitor cells in bone marrow, and model B implemented shortening of differentiation and maturation times of early progenitors in bone marrow. Model C was the combination of models A and B, and model D was the combination of model A with an increase in the maturation times of the circulating reticulocytes. Model evaluation was performed using goodness-of-fit plots, a nonparametric bootstrap and a posterior predictive check.
Results: Model D was selected as the best model, and evidenced accurate and precise estimation of model parameters and prediction of the time course of the percentage of reticulocytes. At baseline, the estimated circulating reticulocyte maturation time was 2.6 days, whereas the lifespan of the precursors in the bone marrow was about 5 days. The rHuEPO potency for the stimulatory effect (7.61 IU/L) was higher than that for the increase in reticulocyte maturation times (56.3 IU/L). There was a significant 1- to 2-day lag time in the reticulocyte response. The effect of rHuEPO on the reticulocyte age distribution consisted of a transient increase in the reticulocyte maturation time from baseline up to 6–7 days, occurring 1 day after administration. The dose-dependent amplitude of the changes in the age distribution lasted for 12–14 days. The model-predicted peak increase in the reticulocyte release rate ranged from 140% to 160% of the baseline value and was maximal on days 7–8 following rHuEPO administration.
Conclusions: A semiphysiological model quantifying the effect of rHuEPO on the reticulocyte production rate and age distribution was developed. The validated model predicts that rHuEPO increases the reticulocyte production rate and modifies the reticulocyte age distribution in a dose-dependent manner.










Similar content being viewed by others
References
Koury MJ, Sawyer ST, Brandt S. New insights into erythropoiesis. Opin Hematol 2002; 9: 93–100
Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med 2003; 228: 1–14
Krantz SB. Erythropoietin. Blood 1991; 77: 419–34
Chang CC, Kass L. Clinical significance of immature reticulocyte fraction determined by automated reticulocyte counting. Am J Clin Pathol 1997; 108: 69–73
Tatsumi N, Izumi T. Reticulocyte maturation index as a useful diagnostic parameter. Sysmes Int 1991; 1: 23–8
Sowade O, Sowade B, Brilla K. Kinetics of reticulocyte maturity fractions and indices and iron status during therapy with epoetin beta (recombinant human erythropoietin) in cardiac surgery patients. Am J Hematol 1997; 55: 89–96
Brugnara C. Reticulocyte cellular indices: a new approach in the diagnosis of anemias and monitoring of erythropoietic function. Crit Rev Clin Lab Sci 2000; 37: 93–130
Major A, Bauer C, Breymann C, et al. rh-Erythropoietin stimulates immature reticulocyte release in man. Br J Haematol 1994; 87: 605–8
Al-Huniti NH, Widness JA, Schmidt RL, et al. Pharmacodynamic analysis of changes in reticulocyte subtype distribution in phlebotomy-induced stress erythropoiesis. J Pharmacokinet Pharmacodyn 2005; 32: 359–76
Krzyzanski W, Pérez-Ruixo JJ. An assessment of recombinant human erythropoietin effect on reticulocyte production rate and lifespan distribution in healthy subjects. Pharm Res 2007; 24: 758–71
Harker LA, Roskos LK, Marzec UM, et al. Effects of megakaryocyte growth and development factor on platelet production, platelet life span, and platelet function in healthy human volunteers. Blood 2000; 95: 2514–22
Roskos LK, Lum P, Lockbaum P, et al. Pharmacokinetic/pharmacodynamic modeling of pegfilgrastim in healthy subjects. J Clin Pharmacol 2006; 46: 747–57
Simeoni M, Magni P, Cammia C, et al. Predictive pharmacokinetic-pharmacodynamic modeling of tumor growth kinetics in xenograft models after administrations of anticancer agents. Cancer Res 2004; 64: 1094–101
Cheung WK, Goon BL, Guilfoyle MC, et al. Pharmacokinetics and pharmacodynamics of recombinant human erythropoietin after single and multiple subcutaneous doses to healthy subjects. Clin Pharmacol Ther 1998; 64: 412–23
Ratnasingam L. An open-label, randomized, parallel design study to investigate the pharmacokinetic and pharmacodynamic profiles of single subcutaneously administered doses of epoetin alfa in healthy male volunteers. Protocol EPOPHI-380. Raritan (NJ): Johnson & Johnson Pharmaceutical Research and Development, 2001. (Data on file)
Grune W. New developments in flow cytochemistry technology. Atlas Automated Cytochem Hematol 1996; 94: 1–6
Holford N. Wings for NONMEM [online]. Available from URL: http://wfn.sourceforge.net [Accessed 2008 Mar 4]
Olsson-Gisleskog P, Jacqmin P, Pérez-Ruixo JJ. Population pharmacokinetics meta-analysis of recombinant human erythropoietin in healthy subjects. Clin Pharmacokinet 2007; 46: 159–73
Zhang L, Beal SL, Sheiner LB. Simultaneous vs sequential analysis for population PK/PD data I: best-case performance. J Pharmacokinet Pharmacodyn 2003; 30: 387–404
Sun Y-N, Jusko WJ. Transit compartments versus gamma distribution function to model signal transduction process in pharmacodynamics. J Pharm Sci 1998; 87: 732–7
Mager DE, Jusko WJ. Pharmacodynamic modeling of time-dependent transduction systems. Clin Pharmacol Ther 2001; 70: 210–6
Efron B, Tibshirani R. An introduction to the bootstrap. London: Chapman and Hall, 1993
Yano Y, Beal SL, Sheiner LB. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn 2001; 28: 171–92
Ramakrishnan R, Cheung WK, Wacholtz MC, et al. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after single and multiple doses in healthy volunteers. J Clin Pharmacol 2004; 44: 991–1002
Krzyzanski W, Jusko WM, Wacholtz MC, et al. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after multiple subcutaneous doses in healthy subjects. Eur J Pharm Sci 2005; 26: 295–306
Pérez-Ruixo JJ, Kimko HC, Chow AT, et al. Population cell life span models for effects of drugs following indirect mechanisms of action. J Pharmacokinet Pharmacodyn 2005; 32: 767–93
Spivak JL. The mechanism of action of erythropoietin. Int J Cell Cloning 1986; 4: 139–66
Bugelski PJ, Nesspor T, Volk A, et al. Pharmacodynamics of recombinant human erythropoietin in murine bone marrow. Pharm Res 2008; 25: 369–78
Trial J, Rice L, Alfrey CP. Erythropoietin withdrawal alters interactions between young red blood cells, splenic endothelial cells, and macrophages: an in vitro model of neocytolysis. J Invest Med 2001; 49: 335–45
Agoram B, Heatherington AC, Gastonguay MR. Development and evaluation of a population pharmacokinetic-pharmacodynamic model of darbepoetin alfa in patients with nonmyeloid malignancies undergoing multicycle chemotherapy. AAPS J 2006 Sep 1;8 (3): E552–63
Wiczling P, Krzyzanski W. Flow cytometric assessment of homeostatic aging of reticulocytes in rats. Exp Hematol 2008; 36: 119–27
Woo S, Krzyzanski W, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after intravenous and subcutaneous administration in rats. J Pharmacol Exp Ther 2006; 319: 1297–306
Acknowledgements
This study was supported by Johnson & Johnson Pharmaceutical Research & Development (a Division of Janssen Pharmaceutica, NV, Beerse, Belgium) and in part by National Institutes of Health grant no. GM 57980 from the National Institute of General Medical Sciences (Bethesda, MD, USA). The study was presented in part at the Annual Meeting of the Population Approach Group in Europe (Copenhagen, 13–15 June 2007). Juan José Pérez-Ruixo is a former employee and Jeremy Hing is a current employee of Johnson & Johnson Pharmaceutical Research & Development. Wojciech Krzyzanski is a consultant for Johnson & Johnson Pharmaceutical Research & Development.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Mean Residence Time for One-Compartment Model with Time-Dependent First-Order Elimination Rate
Consider a one-compartment model where drug amount A is eliminated at the first-order rate k(t), which can depend on time (equation 35):

Let t0 be an arbitrary time and the amount of drug at this time is A(t0). For an incrementally small Δt, the number of drug particles comprising A(t0) that exit the compartment between times t and t + Δt is k(t) · (A(t)/m) · Δt, where m is the mass of a single drug particle. In addition, let tout denote the time at which a particle exits the compartment. Then tout can be considered as a random variable, and the probability that a particle will leave the compartment between times t and t + Δt can be calculated as equation 36:
Consequently, the probability density function for the tout distribution is represented by equation 37:
 and the expected tout [E(tout)] can be expressed as follows (equation 38):
and the expected tout [E(tout)] can be expressed as follows (equation 38):

The time during which a particle of A(t0) resides in the compartment is the time elapsing between t0 and tout. Hence the MRT is (equation 39):

To simplify the integral in equation 38, multiply both sides of equation 35 by t and integrate from t0 to infinity (equation 40):

Integration by parts implies that (equation 41):

Combining equations 38 and 41 results in equation 42:
Note that introducing a new variable X(t, t0) = A(t)/A(t0) [equation 43]:
 where X(t, t0) is a solution to equation 44:
where X(t, t0) is a solution to equation 44:
 with the initial condition (equation 45):
with the initial condition (equation 45):

Rights and permissions
About this article
Cite this article
Pérez-Ruixo, J.J., Krzyzanski, W. & Hing, J. Pharmacodynamic Analysis of Recombinant Human Erythropoietin Effect on Reticulocyte Production Rate and Age Distribution in Healthy Subjects. Clin Pharmacokinet 47, 399–415 (2008). https://doi.org/10.2165/00003088-200847060-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200847060-00004