Summary
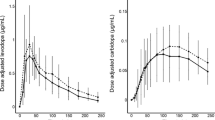
Studies on the concentration-effect relationship of levodopa in Parkinson’s disease have established that: (1) in patients with a fluctuating response to levodopa, concentration-effect profiles are steeper and markedly shifted to the right (i.e. potency is decreased) compared with those patients whose symptoms are adequately controlled; (2) with controlled-release (CR) preparations, the concentration-effect relationship indicates a decreased potency compared with conventional immediate-release (IR) preparations; and (3) coadministration of a dopamine receptor agonist (even at a subclinical dose) enhances the potency of levodopa. These findings support some current hypotheses on the origin of, and the pathophysiological process underlying, response fluctuations.
In patients with response fluctuations, metabolism of levodopa and storage of dopamine in the striatum are reduced. Levodopa is decarboxylated in the extracellular space, with the result that dopamine is released directly to the effect site. Thus, without dopamine storage acting as a buffer between levodopa metabolism and dopaminergic effect, the decline in motor response closely follows the decrease in levodopa concentrations. Even small fluctuations of levodopa concentrations around the EC50 value (the concentration threshold necessary to produce a motor response) might be followed by response fluctuations.
Patients with Parkinson’s disease who do not have response fluctuations exhibit a residual capacity of production and storage of endogenous dopamine; thus, lower amounts of ‘exogenous’ dopamine (formed by decarboxylation of levodopa) are required. The storage buffer is responsible for a time lag between decline in peripheral plasma concentrations of levodopa and dopamine-induced motor response. Low doses of a dopamine receptor agonist increase the basal tonus of the striatum, but do not reach the threshold concentration for triggering a motor response. Because of the dichotomic character of the motor response, patients do not switch from an ‘off’ (not responding) phase to an ‘on’ (responding) phase. However, lower amounts of exogenous dopamine released in the synaptic cleft will be necessary to induce response.
To date, pharmacokinetic-pharmacodynamic modelling does not give a clear answer as to whether response fluctuations are additionally induced by receptor desensitisation or inhibition of the active transport of levodopa across the blood-brain barrier by the main metabolite of levodopa, 3-0-methyldopa. Nevertheless, there is some evidence that higher plasma concentrations of levodopa are required for similar motor effects when CR preparations are compared with IR preparations.
Attempts have been made to establish therapeutic drug monitoring of levodopa in patients with response fluctuations. The interindividual variability of EC50 values in single studies is relatively low (10% to a maximum of 50%), which might allow specification of a ‘population’ threshold plasma concentration (i.e. a minimal effective plasma concentration required to obtain clinical effects). However, considering the short elimination half-life of levodopa, it seems doubtful whether such target drug concentrations can be maintained as steady-state. A marked prolongation of the dosage interval with CR preparations might be limited by the higher threshold concentrations of levodopa necessary to maintain clinical effects.
Similar content being viewed by others
References
Cedarbaum JM. Clinical pharmacokinetics of anti-Parkinsonian drugs. Clin Pharmacokinet 1987; 13: 141–78
Wachtel H. Antiparkinsonian dopamine agonists: a review of the pharmacokinetics and neuropharmacology in animals and humans. J Neural Trans 1991; 3: 151–201
Seeman P, Grigoriadis D. Dopamine receptors in brain and periphery. J Neurochem Int 1987; 1: 1–25
Sage JI, Mark MH. Basic mechanisms of motor fluctuations. Neurology 1994; 44 Suppl. 6: S10–S14
Kempster PA, Frankel JP, Bovingdon M, et al. Levodopa peripheral pharmacokinetics and duration of motor response in parkinson’s disease. J Neurol Neurosurg Psychiatry 1989; 52: 718–23
Cedarbaum JM, Kutt H, McDowell FH. Clinical significance of the relationship between O-methyldopa levels and levodopa intake. Neurology 1988; 38: 533–6
Gancher ST, Nutt JG, Woodward WR. Peripheral pharmacokinetics of levodopa in untreated, stable and fluctuating parkinsonian patients. Neurology 1987; 37: 940–4
Fabbrini G, Mouradian MM, Juncos JL, et al. Motor fluctuations in Parkinson’s disease: central pathophysiological mechanisms, part I. Ann Neurol 1988; 24: 366–71
Mouradian MM, Juncos JL, Fabbrini G, et al. Motor fluctuations in Parkinson’s disease: central pathophysiological mechanisms, part II. Ann Neurol 1988; 24: 372–8
Schelosky L, Poewe W. Current strategies in the drug treatment of advanced Parkinson’s disease — new modes of dopamine substitution. Acta Neurol Scand 1993; 87 Suppl. 146: 46–9
Mouradian MM, Heuser IJE, Baronti F, et al. Modification of central dopaminergic mechanism by continuous levodopa therapy for advanced Parkinson’s disease. Ann Neurol 1990; 27: 18–23
Sage JI, Mark MH. The rationale for continuous dopaminergic stimulation in patients with Parkinson’s disease. Neurology 1992; 42 Suppl. 1: 23–8
Wooten GF. Progress in understanding the pathophysiology of treatment related fluctuations in Parkinson’s diesease. Ann Neurol 1988; 24: 363–5
Baas H, Demisch L, Harder S, et al. Levodopa Resorption in verschiedenen Stadien der Parkinson-Krankheit. In: Fischer PA, editor. Parkinson-Krankheit. Verlaufsbezogene Diagnostik und Therapie. Basel: Editiones Roche, 1993: 97–115
Bredberg E, Tedroff J, Aquilonius SM, et al. Pharmacokinetics and effects of levodopa in advanced Parkinson’s Disease. Eur J Clin Pharmacol 1990; 39: 385–9
Contin M, Riva R, Martinelli P, et al. Pharmacodynamic modeling of oral levodopa. Neurology 1993; 43: 367–71
Nutt JG, Carter JH, Woodward WR. Effect of brief levodopa holiday on the short-duration response to levodopa. Neurology 1994; 44: 1617–22
Cedarbaum JM, Silvestri M, Kutt H. Sustained enterai administration of levodopa increases and interrupted infusion decreases levodopa dose requirements. Neurology 1990; 40: 995–7
Turjanski N, Fernandez W, Lees AJ. The effects of acute levodopa withdrawal on motor performance and dopaminergic receptor sensitivity in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 1993; 56: 771–5
Gervas J, Muradas B, Bazan E, et al. Effects of 3-OM dopa on monoamino metabolism in rat brain. Neurology 1983; 33: 278–82
Calne DB, Reid JL, Vakil SD. Parkinsonism treated with 3-0-methyldopa. Clin Pharmacol Ther 1972; 14: 386–9
Nutt JG, Woodward WR, Carter JH. Clinical and biochemical studies with controlled release levodopa/carbidopa. Neurology 1986; 36: 1206–11
Mena MA, Muradas VI, Bazan E, et al. Pharmacokinetics of L-dopa in patients with Parkinson’s disease. Adv Neurol 1986; 45: 481–6
Bowes SG, O’Neill CJA, Nicholson PW, et al. Effect of duration of levodopa/decarboxylase inhibitor therapy on the pharmacokinetic handling of levodopa in elderly patients with idiopathic Parkinson’s disease. Eur J Clin Pharmacol 1991; 41: 459–62
Weiner WJ, Koller WC, Perlik S, et al. Drug holiday and management of Parkinson’s disease. Neurology 1990; 30: 1257–61
Nutt JG, Woodward WR, Gancher ST, et al. 3-O-methyldopa and the reponse to levodopa in Parkinson’s disease. Ann Neurol 1987; 21: 584–8
Cedarbaum JM, Hoey M, McDowell FH. A double blind crossover comparison of Sinemet CR4 and standard Sinemet 25/100 in patients with Parkinson’s disease and fluctuating motor performance. J Neurol Neurosurg Psychiatry 1989; 52: 207–12
Feldman RG, Mosbach PA, Kelly MR, et al. Double-blind comparison of standard Sinemet and Sinemet CR in patients with mild-to-moderate Parkinson’s disease. Neurology 1989; 39 Suppl. 2: 96–101
Poewe WH, Lees AJ, Stern GM. Clinical and pharmacokinetic observations with Madopar HBS in hospitalized patients with Parkinson’s disease and motor fluctuations. Eur Neurol 1987; 27 Suppl. 1: 93–7
Fischer PA, Baas H. Preliminary experience with Madopar HBS: clinical observations and plasma levodopa concentrations. Eur Neurol 1987; 27 Suppl. 1: 81–7
Ceballos-Baumann AO, von Kummer R, Eckert W, et al. Controlled-release Levodopa/Benserazid (Madopar HBS): clinical observations and Levodopa and Dopamine plasma concentrations in fluctuating Parkinsonian patients. J Neurol 1990; 237: 24–8
Pacchetti C, Martignoni E, Sibilla L, et al. Effectiveness of Madopar HBS plus Madopar standard in patients with fluctuating Parkinson’s disease: two years of follow-up. Eur Neurol 1990; 30: 319–23
Holford NHG, Sheiner LB. Understanding the dose effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin Pharmacokinet 1981; 6: 429–53
Campbell DB. The use of kinetic-dynamic interactions in the evaluation of drugs. Psychopharmacology 1990; 16: 433–50
Verotta D, Beal SL, Sheiner LB. Semiparametric approach to pharmacokinetic-pharmacodynamic data. Am J Physiol 1989; 25: 1005–10
Hochhaus G, Möllmann H. Pharmacokinetic/pharmacodynamic characteristics of the β-2-agonist terbutaline, salbutamol and fenoterol. Int J Clin Pharmacol Ther Toxicol 1992; 30: 342–62
Olanow CW, Gauger LL, Cedarbaum JM. Temporal relationships between plasma and cerebrospinal fluid pharmacokinetics of levodopa and clinical effects in parkinson’s disease. Ann Neurol 1991; 29: 556–9
Jamieson PW. A computational model of Levodopa pharmacodynamics in Parkinson’s disease. Clin Neuropharmacol 1991; 14: 498–513
Nelson MV, Berchou RC, LeWitt PA, et al. Pharmacodynamic modeling of concentration-effect relationships after controlled release carbidopa/levodopa in Parkinson’s disease. Neurology 1990; 40: 70–4
Harder S, Baas H, Bergemann N, et al. Concentration/effect relationship of levodopa in patients with Parkinsons’s disease after oral administration of an immediate release and a controlled release formulation. Br J Clin Pharmacol 1995; 39: 39–44
Harder S, Baas H, Bürcklin F, et al. PK-PD relationship of levodopa alone and under apomorphine in patients with Parkinsons’s disease [abstract]. Clin Pharmacol Ther 1995; 57: 145
Dayneka NL, Garg V, Jusko WJ. Comparison of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharmaceut 21: 457–78
Leger G, Reches A, Cedarbaum J, et al. Inhibition of 3-O-methylfluorodopa formation with OR-462. Neurology 1990; 40 Suppl. 1: 156
Bredberg E, Nilsson D, Johansson K, et al. Intraduodenal infusion of a water-based levodopa dispersion for optimisation of the therapeutic effect in severe Parkinson’s disease. Eur J Clin Pharmacol 1993; 45: 117–22
Kurlan R, Rubin AJ, Miller C, et al. Duodenal delivery of levodopa for on-off fluctuations in parkinsonism: preliminary observations. Ann Neurol 1986; 20: 262–5
Spector R, Park GD, Johnson GF, et al. Therapeutic drug monitoring. Clin Pharmacol Ther 1988; 43: 345–53
Poewe W, Kleedorfer B, Gerstenbrand F, et al. Die Behandlung von Parkinsonpatienten mit L-Dopa-Wirkungsfluktuation mittels subkutanen Apomorphingaben. Aktuel Neurol 1989; 16: 73–7
Stibe CMH, Lees AJ, Kempster PA, et al. Subcutanous apomeorphine in parkinsonian on-off oscillations. Lancet 1989; i: 403–6
Myllylä VV, Sotaniemi KA, Illi A, et al. Effect of entacapone, a COMT inhibitor, on the pharmacokinetics of levodopa and on cardiovascular responses in patients with Parkinson’s disease. Eur J Clin Pharmacol 1993; 45: 419–23
Leenders KL, Salmom EP, Turton DR, et al. The nigrostriatal dopaminergic system assessed in vivo by positron emission tomography in healthy volunteers and patients with Parkinsons’s disease. Arch Neurol 1990; 30: 24–30
Unadkat JD, Bartha F, Sheiner LB. Simultanous modeling of pharmacokinetics and pharmacodynamics with nonparametric kinetic and dynamic models. Clin Pharmacol Ther 1986; 36: 86–93
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Harder, S., Baas, H. & Rietbrock, S. Concentration-Effect Relationship of Levodopa in Patients with Parkinson’s Disease. Clin-Pharmacokinet 29, 243–256 (1995). https://doi.org/10.2165/00003088-199529040-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199529040-00004