Abstract
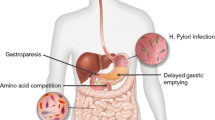
Levodopa (LD) is the most effective medication to treat Parkinson’s disease (PD). However, motor fluctuations and drug-induced dyskinesia compromise the long-term success of levodopa therapy in PD. These response complications are due, at least in part, to fluctuating LD plasma levels (as a result of erratic gastric emptying, variable jejunal absorption, and most importantly, the short half-life of LD) with standard levodopa formulations. Keeping levodopa concentrations as constant as possible is the target for improving the pharmacokinetics and developing new ways of LD administration. In this article, we review novel oral and non-oral LD formulations including the ones that have successfully completed phase 3 clinical trials and have come to market and ones that are still in earlier phases of clinical development.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–6.
Deleu D, Northway MG, Hanssens Y. Clinical pharmacokinetic and pharmacodynamic properties of drugs used in the treatment of Parkinson’s disease. Clin Pharmacokinet. 2002;41(4):261–309.
Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson’s disease: scientific rationale and clinical implications. Lancet Neurol. 2006;5(8):677–87.
Quinn N, Marsden CD, Parkes JD. Complicated response fluctuations in Parkinson’s disease: response to intravenous infusion of levodopa. Lancet. 1982;2(8295):412–5.
Hardie RJ, Malcolm SL, Lees AJ, Stern GM, Allen JG. The pharmacokinetics of intravenous and oral levodopa in patients with Parkinson’s disease who exhibit on-off fluctuations. Br J Clin Pharmacol. 1986;22(4):429–36.
Chase TN, Baronti F, Fabbrini G, Heuser IJ, Juncos JL, Mouradian MM. Rationale for continuous dopaminomimetic therapy of Parkinson’s disease. Neurology. 1989;39(11 Suppl 2):7–10. discussion 9.
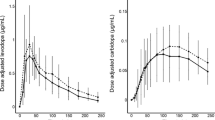
Mao Z, Hsu A, Gupta S, Modi NB. Population pharmacodynamics of IPX066: an oral extended-release capsule formulation of carbidopa-levodopa, and immediate-release carbidopa-levodopa in patients with advanced Parkinson’s disease. J Clin Pharmacol. 2013;53(5):523–31.
Pahwa R, Lyons KE, Hauser RA, Fahn S, Jankovic J, Pourcher E, et al. Randomized trial of IPX066, carbidopa/levodopa extended release, in early Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(2):142–8.
Hauser RA, Hsu A, Kell S, Espay AJ, Sethi K, Stacy M, et al. Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson’s disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurol. 2013;12(4):346–56. Phase III clinical trial showing reduced OFF time and increased ON time with IPX066 in comparison to immediate release CD-LD in advanced PD.
Stocchi F, Hsu A, Khanna S, Ellenbogen A, Mahler A, Liang G, et al. Comparison of IPX066 with carbidopa-levodopa plus entacapone in advanced PD patients. Parkinsonism Relat Disord. 2014;20(12):1335–40. Phase III clinical trial showing reduced OFF time and increased ON time with IPX066 in comparison to immediate release CD-LD plus entacapone in advanced PD.
Waters CH, Nausieda P, Dzyak L, Spiegel J, Rudzinska M, Silver DE, et al. Long-term treatment with extended-release carbidopa-levodopa (IPX066) in early and advanced Parkinson’s disease: a 9-month open-label extension trial. CNS Drugs. 2015;29(4):341–50.
Lewitt PA, Ellenbogen A, Chen D, Lal R, McGuire K, Zomorodi K, et al. Actively transported levodopa prodrug XP21279: a study in patients with Parkinson disease who experience motor fluctuations. Clin Neuropharmacol. 2012;35(3):103–10.
LeWitt PA, Huff FJ, Hauser RA, Chen D, Lissin D, Zomorodi K, et al. Double-blind study of the actively transported levodopa prodrug XP21279 in Parkinson’s disease. Mov Disord. 2014;29(1):75–82.
Chen C, Cowles VE, Sweeney M, Stolyarov ID, Illarioshkin SN. Pharmacokinetics and pharmacodynamics of gastroretentive delivery of levodopa/carbidopa in patients with Parkinson disease. Clin Neuropharmacol. 2012;35(2):67–72.
Hou SY, Cowles VE, Berner B. Gastric retentive dosage forms: a review. Crit Rev Ther Drug Carrier Syst. 2003;20(6):459–97.
Verhagen Metman L, Stover N, Chen C, Cowles VE, Sweeney M. Gastroretentive carbidopa/levodopa, DM-1992, for the treatment of advanced Parkinson’s disease. Mov Disord. 2015;30(9):1222–8. Phase II trial demonstrating drug pharmacokinetics.
LeWitt PA, Giladi N, Gurevich T, et al. Accordion pill carbidopa/ levodopa (AP CD/LD) for treatment of advanced Parkinson’s disease (PD). Mov Disord. 2014;29 suppl 1:S248.
Nyholm D, Lennernas H. Irregular gastrointestinal drug absorption in Parkinson’s disease. Expert Opin Drug Metab Toxicol. 2008;4(2):193–203.
Nilsson D, Hansson LE, Johansson K, Nystrom C, Paalzow L, Aquilonius SM. Long-term intraduodenal infusion of a water based levodopa-carbidopa dispersion in very advanced Parkinson’s disease. Acta Neurol Scand. 1998;97(3):175–83.
Antonini A, Isaias IU, Canesi M, Zibetti M, Mancini F, Manfredi L, et al. Duodenal levodopa infusion for advanced Parkinson’s disease: 12-month treatment outcome. Mov Disord. 2007;22(8):1145–9.
Eggert K, Schrader C, Hahn M, Stamelou M, Russmann A, Dengler R, et al. Continuous jejunal levodopa infusion in patients with advanced Parkinson disease: practical aspects and outcome of motor and non-motor complications. Clin Neuropharmacol. 2008;31(3):151–66.
Fernandez HH, Odin P. Levodopa-carbidopa intestinal gel for treatment of advanced Parkinson’s disease. Curr Med Res Opin. 2011;27(5):907–19.
Nilsson D, Nyholm D, Aquilonius SM. Duodenal levodopa infusion in Parkinson’s disease—long-term experience. Acta Neurol Scand. 2001;104(6):343–8.
Nyholm D. Enteral levodopa/carbidopa gel infusion for the treatment of motor fluctuations and dyskinesias in advanced Parkinson’s disease. Expert Rev Neurother. 2006;6(10):1403–11.
Samanta J, Hauser RA. Duodenal levodopa infusion for the treatment of Parkinson’s disease. Expert Opin Pharmacother. 2007;8(5):657–64.
Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13(2):141–9. Phase III clinical trial showing reduced OFF time and increased ON time without troublesome dyskinesia with LCIG in comparison to immediate release CD-LD.
Nyholm D. Duodopa(R) treatment for advanced Parkinson’s disease: a review of efficacy and safety. Parkinsonism Relat Disord. 2012;18(8):916–29.
Jugel C, Ehlen F, Taskin B, Marzinzik F, Muller T, Klostermann F. Neuropathy in Parkinson’s disease patients with intestinal levodopa infusion versus oral drugs. PLoS One. 2013;8(6):e66639.
Toth C, Brown MS, Furtado S, Suchowersky O, Zochodne D. Neuropathy as a potential complication of levodopa use in Parkinson’s disease. Mov Disord. 2008;23(13):1850–9.
Sensi M, Preda F, Trevisani L, Contini E, Gragnaniello D, Capone JG, et al. Emerging issues on selection criteria of levodopa carbidopa infusion therapy: considerations on outcome of 28 consecutive patients. J Neural Transm (Vienna). 2014;121(6):633–42.
Hauser RA. Future treatments for Parkinson’s disease: surfing the PD pipeline. Int J Neurosci. 2011;121 Suppl 2:53–62.
Caraco Y, Oren S, LeWitt P. Constant therapeutic levodpa (LD) plasma concentrations maintained by continuous subcutaneous (SC) administration of ND-0612, a novel formulation of LD/carbidopa (CD). Mov Disord. 2013;28:S162.
Caraco Y, Oren S, Yacoby-Zeevi O, et al. ND0612, a novel formulation of levodopa/carbidopa for continuous, subcutaneous administration, achieves steady-state levodopa plasma concentration in Parkinson’s disease patients. Mov Disord. 2013;79:56.
Oren S, Yacobi-Zeevi O, Cohen Y, Djaldetti R, Gurevich T, Caraco Y, et al. Pharmacokinetic profile of ND0612L (levodopa/carbidopa for subcutaneous infusion) in patients with moderate to severe Parkinson’s disease [abstract]. Mov Disord. 2015;30 Suppl 1:226.
Poewe W, Antonini A. Novel formulations and modes of delivery of levodopa. Mov Disord. 2015;30(1):114–20. A comprehensive review of the available and experimental novel preparations of levodopa.
Freed MI, Batycky R, Merica E. Safety, tolerability and levodopa pharmacokinetics following inhaled administration of CVT-301, a levodopa dry powder aerosol, in healthy, adult subjects [abstract]. Mov Disord. 2013;28 Suppl 1:430.
LeWitt PA, Saint-Hilaire MH, Grosset DG, Hauser R, Stocchi F, Freed MI, et al. Inhaled levodopa (CVT‐301) provides rapid motor improvements after administration to Parkinson’s disease patients when OFF [abstract]. Mov Disord. 2015;30 Suppl 1:260.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Yasaman Kianirad declares no conflict of interest.
Tanya Simuni has received consultancy fees from Abbvie, Harbor, Merz Inc., Navidea, UCB Pharma, US World Meds, Acadia, and Eli Lilly, speaker and consultation fees from Allergan and Lundbeck, and speaker, consultation fees, and honorarium from GE Medical and Ibsen. Dr. Simuni has also received advisory board consultation fees and research funding from IMPAX, research funding from Auspex, Biotie, and Civitas as well as a grant and consultation fees from the National Parkinson Foundation, speaker honorarium, consultant research and education grants from TEVA, and grants from the NIH and Michael J. Fox Foundation.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Movement Disorders
Rights and permissions
About this article
Cite this article
Kianirad, Y., Simuni, T. Novel Approaches to Optimization of Levodopa Therapy for Parkinson’s Disease. Curr Neurol Neurosci Rep 16, 34 (2016). https://doi.org/10.1007/s11910-016-0635-8
Published:
DOI: https://doi.org/10.1007/s11910-016-0635-8