Abstract
Objectives
To compare persistency rates and persistency days in patients with Alzheimer’s disease (AD) who initiated therapy with either rivastigmine or donepezil, and to identify factors influencing persistency in a real-world setting.
Design and methods
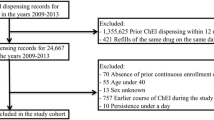
This study used data collected by MarketScan® from 1 January 1999 to 31 December 2002. Patients were included if they were newly diagnosed with AD and filled at least one prescription for rivastigmine or donepezil between 1 July 2000 and 30 June 2001, were ≥65 years of age on the index prescription date, and had continuous health and prescription insurance during the entire study period. Patients were excluded if they filled a prescription for any cholinesterase inhibitor during the 18 months prior to initiation of the study drugs. Patients who refilled their initial cholinesterase inhibitor prescription within a permissible gap of 60 days after depleting the drug supply from the prior prescription were considered to be persistent. Sensitivity analysis was performed to test the robustness of the persistency definition. The Kaplan-Meier method was used to determine persistency rates across time and Cox proportional hazards models were used to estimate relative risks of discontinuation or switch with adjustment for other covariates, and to identify factors significantly influencing persistency of the study drugs.
Results
Of the newly treated AD patients, the proportion of rivastigmine and donepezil patients who continued their medication was the same (47%; p = 0.5). On average, rivastigmine users continuously used their medication for 234 days (median 312 days) while those taking donepezil used their medication for 235 days (median 315 days) [p = 0.91]. Patients were more likely to discontinue or switch their initial cholinesterase inhibitor if they used a central nervous system (CNS) medication before initiation of therapy (relative risk [RR] = 1.23; 95% CI 1.01, 1.51 without adjustment for study variables; RR = 1.30; 95% CI 1.05, 1.60 with adjustment for study variables). On the other hand, patients were less likely to discontinue their cholinesterase inhibitor if they visited their physician office frequently (RR = 0.24; 95% CI 0.18, 0.32 without adjustment; RR = 0.23; 95% CI 0.17, 0.30 with adjustment) or if they were hospitalised after initiation of their cholinesterase inhibitor therapy (RR = 0.60; 95% CI 0.39, 0.91 without adjustment; RR = 0.65; 95% CI 0.42, 0.99 with adjustment).
Conclusion
Patients who were newly diagnosed with AD and initiated therapy with either rivastigmine or donepezil had similar levels of persistency with their initial AD therapy in a real-world setting.






Similar content being viewed by others
References
Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol 2003; 60: 1119–22
Ernst R, Hay J. Economic research on Alzheimer disease: a review of the literature. Alzheimer Dis Assoc Disord 1997; 11Suppl. 6: 135–45
Cummings JL. Guidelines for managing Alzheimer’s disease: part II. Treatment. Am Fam Physician 2002; 65(12): 2525–34
Ballard C. Advances in the treatment of Alzheimer’s disease: benefits of dual cholinesterase inhibition. Eur Neurol 2002; 47: 64–70
Rogers S, Doody R, Pratt R, et al. Long-term efficacy and safety of donepezil in the treatment of Alzheimer’s disease: final analysis of a US multicentre open-label study. Eur Neuropsy-chopharmacol 2000; 10: 195–203
Raskind M, Peskind E, Wessel T, et al. Galantamine in AD: a 6-month, randomized, placebo-controlled trial with a 6-month extension. Neurology 2000; 54: 2261–8
Farlow M, Anand R, Messina JJ, et al. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s disease. Eur Neurol 2000; 44: 236–41
Farlow MR. Do cholinesterase inhibitors slow progression of Alzheimer’s disease? Int J Clin Pract Suppl 2002; (127): 37–44
Neumann PJ, Hermann RC, Kuntz KM, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer’s disease. Neurology 1999; 52(6): 1138–45
Lamb HM, Goa KL. Rivastigmine, a pharmacoeconomic review of its use in Alzheimer’s disease. Pharmacoeconomics 2001; 19(3): 303–18
Wolfson C, Oremus M, Shukla V, et al. Donepezil and rivastigmine in the treatment of Alzheimer’s disease: a best-evidence synthesis of the published data on their efficacy and cost-effectiveness. Clin Ther 2002; 24(6): 862–86
Hauber AB, Gnanasakthy A, Snyder EH, et al. Potential savings in the cost of caring for Alzheimer’s disease: treatment with rivastigmine. Pharmacoeconomics 2000; 17(4): 351–60
National Institute for Clinical Excellence. Appraisal consultation document. Alzheimer’s disease: donepezil, rivastigmine, galantamine and memantine. London: National Institute for Clinical Excellence, 2005
Auriacombe S, Pere J, Joria-Kanza Y, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease who failed to benefit from treatment with donepezil. Curr Med Res Opin 2002; 18(3): 129–38
Bullock R, Connolly C. Switching cholinesterase inhibitor therapy in Alzheimer’s disease: donepezil to rivastigmine, is it worth it? Int J Geriatr Psychiatry 2002; 17: 288–9
Emre M. Switching cholinesterase inhibitors in patients with Alzheimer’s disease. Int J Clin Pract Suppl 2002; 127: 64–72
Auriacombe S, Pere J-J. No donepezil discontinuation effect in patients with Alzheimer’s disease who were switched to rivastigmine after failing to benefit from donepezil treatment. Curr Med Res Opin 2003; 19(8): 715–7
Andrade SE, Walker AM, Gottlieb LK, et al. Discontinuation of antihyperlipidemic drugs: do rates reported in clinical trials reflect rates in primary care setting? N Engl J Med 1995; 332(17): 1125–31
Schwartz GF. Measuring persistency with drug therapy in glaucoma management. J Managed Care Pharm 2002; 8(10): S237–S9
Dasgupta S, Oates V, Bookhart B, et al. Population-based persistency rates for topical glaucoma medications measured with pharmacy claims data. J Manag Care Pharm 2002; 8: S255–S61
Hanlon J, Fillenbaum G, Burchett B, et al. Drug-use patterns among black and nonblack community-dwelling elderly. Ann Pharmacother 1992; 26: 679–85
Rathore S, Mehta S, Boyko W, et al. Prescription medication use in older Americans: a national report card on prescribing. Fam Med 1998; 30: 733–9
Spiker EC, Emptage RE, Giannamore MR, et al. Potential adverse drug events in an indigent and homeless geriatric population. Ann Pharmacother 2001; 35: 1166–72
Dailey G, Kim MS, Lian JF. Patient compliance and persistence with antihyperglycemic drug regimens: evaluation of a Medicaid patient population with type 2 diabetes mellitus. Clin Ther 2001; 23(8): 1311–20
Catalan VS, LeLorier J. Predictors of long-term persistence on statins in a subsidized clinical population. Value Health 2000; 3(6): 417–26
Reardon G, Schwartz GF, Mozaffari E. Patient persistency with ocular prostaglandin therapy: a population-based, retrospective study. Clin Ther 2003; 25(4): 1172–85
Desgagne A, Lelorier J. Incontinence drug utilization patterns in Quebec, Canada. Value Health 1999; 2(6): 452–8
Suh D-C, Arcona S, Thomas SK, et al. Risk of antipsychotic drug use in patients with Alzheimer’s disease treated with rivastigmine. Drug Aging 2004; 21(6): 395–403
Gambassi G, Landi F, Lapane KL, et al. Predictors of mortality in patients with Alzheimer’s disease living in nursing homes. J Neurol Neurosurg Psychiatry 1999; 67: 59–65
Weiner M, Powe NR, Weiler WE, et al. Alzheimer’s disease under managed care: implications from Medicare utilization and expenditure patterns. J Am Geriatr Soc 1998; 46(6): 762–70
Collett D. Modelling survival data in medical research. New York: Chapman and Hall, 1994
Wilkinson D, Passmore A, Bullock R, et al. A multinational, randomised, 12-week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer’s disease. Int J Clin Pract 2002; 56(6): 441–6
Conlin PR, Gerth WC, Fox J, et al. Four-year persistence patterns among patients initiating therapy with the angiotensin II receptor antagonist losartan versus other antihypertensive drug classes. Clin Ther 2001; 23(12): 1999–2010
Roe CM, Anderson MJ, Spivack B. How many patients complete an adequate trial of donepezil? Alzheimer Dis Assoc Disord 2002; 16(1): 49–51
Jeste SD, Patterson TL, Palmer BW, et al. Cognitive predictors of medication adherence among middle-aged and older outpatients with schizophrenia. Schizophr Res 2002; 63: 49–58
DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment. Arch Intern Med 2000; 160: 2101–7
Gurwitz JH, Yeomans SM, Glynn RJ, et al. Patient noncompliance in the managed care setting: the case of medical therapy for glaucoma. Med Care 1998; 36(3): 357–69
Bailey JE, Lee MD, Somes GW, et al. Risk factors for antihypertensive medication refill failure by patients under Medicaid managed care. Clin Ther 1996; 18(6): 1252–62
Bull SA, Hu XH, Hunkeler EM, et al. Discontinuation of use and switching of antidepressants. JAMA 2002; 288(11): 1403–9
Shaya FT, Mullins DC, Wong W, et al. Discontinuation rates of topical glaucoma medications in a managed care population. J Managed Care Pharm 2002; 8 (10 Suppl.): S271–7
Geldmacher DS, Provenzano G, McRae T, et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s disease. J Am Geriatr Soc 2003; 51: 937–44
Hill J, Futterman R, Mastey V, et al. The effect of donepezil therapy on health costs in a Medicare managed care plan. Manag Care Interface 2002; 15(3): 63–70
Rogers SL, Farlow MR, Doody RS, et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology 1998; 50: 136–45
Moretti R, Torre P, Antonello RM, et al. Rivastigmine in subcortical vascular dementia: a comparison trial on efficacy and tolerability for 12 months follow-up. Eur J Neurol 2001; 8: 361–2
Farlow M, Potkin S, Koumaras B, et al. Analysis of outcome in retrieved dropout patients in a rivastigmine vs placebo, 26-week, Alzheimer Disease trial. Arch Neurol 2003; 60: 843–8
Acknowledgements
The study was partially funded by an unrestricted research grant from Novartis Pharmaceuticals Corporation.
The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suh, DC., Thomas, S.K., Valiyeva, E. et al. Drug Persistency of Two Cholinesterase Inhibitors. Drugs Aging 22, 695–707 (2005). https://doi.org/10.2165/00002512-200522080-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002512-200522080-00006