Summary
Abstract
Brinzolamide is a highly specific carbonic anhydrase (CA) inhibitor which lowers intraocular pressure (IOP) by reducing the rate of aqueous humour formation. Formulated as a 1 % ophthalmic suspension (Azopt®) and administered twice or three times daily, brinzolamide is indicated for the topical management of primary open-angle glaucoma (POAG) and ocular hypertension (OH) as either monotherapy or adjunctive therapy with topical β-blockers.
As monotherapy in patients with POAG or OH, brinzolamide 1% demonstrated IOP-lowering efficacy that was significantly greater than placebo, equivalent to three-times-daily dorzolamide 2% but significantly lower than twice-daily timolol 0.5%. Brinzolamide 1% was equally effective in twice- and three-times—daily regimens producing diurnal mean IOP reductions from baseline in the range of 13.2–21.8%. When used adjunctively twice daily with timolol 0.5%, brinzolamide 1% was as effective as dorzolamide 2% and superior to placebo in lowering IOP in patients with POAG or OH.
In clinical trials, brinzolamide 1% was well tolerated causing only nonserious adverse effects that were generally local, transient and mild to moderate in severity. The incidence of the most common adverse events associated with the use of brinzolamide 1 % was either similar to (blurred vision and abnormal taste) or significantly lower than (ocular discomfort) with dorzolamide 2%. Topical brinzolamide 1% does not appear to produce the acid-base or electrolyte disturbances and severe systemic adverse effects characteristic of oral CA inhibitors. It can be used in patients unresponsive to β-blockers or in whom β-blockers are contraindicated.
Brinzolamide 1% administered twice daily is among the least costly alternatives and adjuncts to β-blocker therapy for glaucoma and is generally associated with less direct medical cost than dorzolamide.
Conclusion: Brinzolamide 1% ophthalmic suspension administered twice or three times daily, as monotherapy or adjunctive therapy with topical β-blockers, has good IOP-lowering efficacy in patients with POAG or OH that is equivalent to that of dorzolamide 2% (three times daily as monotherapy, twice daily as adjunctive therapy). Brinzolamide is generally well tolerated and does not produce the systemic adverse effects associated with oral CA inhibitors. It can be used in patients who are unresponsive to, intolerant of, or unable to receive, ophthalmic β-blockers. Thus, brinzolamide, either as monotherapy or adjunctive therapy with topical β-blockers, should be regarded as a good second-line option in the pharmacological management of POAG and OH, and may be preferred over dorzolamide because of significantly less ocular discomfort.
Overview of Pharmacodynamic Properties
Brinzolamide is a nonbacteriostatic sulfonamide derivative with higher lipophilicity and lower aqueous solubility than dorzolamide or acetazolamide at physiological pH. As a consequence, brinzolamide forms a suspension at pH 7.4 which is more comfortable to the eye than the acidic pH of dorzolamide solution (pH 5.6).
Brinzolamide is a highly specific, noncompetitive and reversible carbonic anhydrase (CA)-II inhibitor with an ≈4-fold greater in vitro binding affinity for CA-II than dorzolamide. The main local ocular effect of brinzolamide is produced predominantly by inhibition of CA-II in the secretory cells of ciliary processes inside the eye. Inhibition of this isoenzyme reduces the rate of aqueous humour formation, consequently lowering intraocular pressure (IOP).
Following topical instillation, brinzolamide enters the blood circulation but systemic adverse effects with its use do not occur mainly because of incomplete saturation and inhibition of CA-II in erythrocytes and kidneys, and the low affinity of brinzolamide for other CA isoforms in the human body.
Brinzolamide has been shown to produce significant reduction in IOP compared with placebo in the eyes of healthy volunteers and patients with glaucoma (see also Therapeutic Efficacy summary). Significantly greater reductions in both daytime and night-time rates of aqueous humour flow were obtained with brinzolamide compared with both placebo (p < 0.001) and dorzolamide (p < 0.05) in healthy volunteers. However, in the same study, the effects on IOP reduction at both trough and peak times were similar with both drugs.
CA-II is also present in corneal endothelium, where it plays a role in the mechanism responsible for maintaining corneal stroma in a relatively dehydrated state. Inhibition of this mechanism can potentially lead to corneal decompensation and impaired vision. Brinzolamide 1% appears to have no adverse effect on corneal endothelial cell function in patients with normal corneas; no clinically relevant or statistically significant changes from baseline in corneal thickness and corneal endothelial cell density occurred in patients with primary open-angle glaucoma (POAG) or ocular hypertension (OH) during an 18-month clinical trial.
Topically applied brinzolamide significantly (p-values not reported) reduced the optic nerve head blood flow to normal levels in the eyes with hypertensive and preperimetric POAG, but caused no change in the eyes with advanced, perimetric glaucoma compared with normal eyes, in a 1-month clinical trial.
Overview of Pharmacokinetic Properties
Published data relating to the pharmacokinetic properties of brinzolamide in humans are limited. After topical ocular instillation in rabbits, brinzolamide 1% was readily absorbed in the conjunctiva, cornea, iris, ciliary body, aqueous humour, lens, choroid and retina, reaching peak concentrations in the anterior eye segment tissues within 0.5–2 hours. Systemic absorption of brinzolamide does occur, but plasma concentrations in healthy human volunteers are generally below quantitation levels because of the drug’s preferential distribution to erythrocytes. Extensive but saturable binding to CA-II in erythrocytes is the cause of nonlinear whole-blood pharmacokinetics of brinzolamide in rats. Concentration-independent plasma protein binding of brinzolamide in the range of 59–63% has been reported in an in vitro study in human plasma.
Metabolic inactivation of brinzolamide is carried out predominantly in the liver through oxidative O- and N-dealkylation by cytochrome P450 isoenzymes. N-desethyl-brinzolamide binds predominantly to CA-I in erythrocytes and is the major metabolite found in whole human blood (but not in plasma). N-desmethox-ypropyl- and O-desmethyl-brinzolamide have been detected as minor metabolites in urine but not in the whole blood of humans.
In humans, brinzolamide is predominantly excreted in the urine (≈60% unchanged and 20% as the N-desethyl metabolite) and has a long whole-blood half-life (111 days following topical administration of brinzolamide 3% ophthalmic suspension three times daily for 14 days in 15 healthy male volunteers).
Therapeutic Efficacy
The efficacy of brinzolamide 1% ophthalmic suspension administered twice or three times daily as monotherapy or adjunctive therapy with twice-daily timolol 0.5% ophthalmic solution has been evaluated in six randomised, double-blind, multicentre, comparative clinical trials of 2 weeks to 18 months duration in a total of 1735 evaluable patients (previously pharmacologically treated) with POAG or OH. The trials compared the IOP-lowering efficacy of brinzolamide 1% with that of dorzolamide 2% (twice daily in monotherapy, three times daily in adjunctive therapy trials), timolol 0.5% (twice daily) or placebo. The primary efficacy endpoint in most trials was the diurnally corrected (i.e. for trough and peak times) mean IOP reduction from baseline; the longest duration trial reported only the mean reduction from baseline in trough IOP (measured at 8am). In all trials, IOP measurements were performed using Goldmann applanation tonometry. All trials were also preceded by appropriate washout periods, which, in two trials evaluating adjunctive use of brinzolamide 1%, also served as run-in phases for nonblind timolol administration.
As Monotherapy: Overall, in four monotherapy trials in a total of 1414 patients, brinzolamide 1% demonstrated IOP-lowering efficacy that was significantly greater than that of placebo (all p < 0.005), similar to dorzolamide 2%, but significantly lower than timolol 0.5% (p < 0.0002, where reported).
In a placebo-controlled, parallel-group trial in 142 patients with POAG or OH, brinzolamide 1–3% produced clinically relevant and statistically significant reductions in mean baseline IOP (p-values not reported). Brinzolamide 1%, 2% and 3% were equally effective in reducing diurnally corrected mean IOP from baseline and more effective than brinzolamide 0.3% (p < 0.036). The study found brinzolamide 1 % to be the optimal concentration for lowering the elevated IOP in patients with glaucoma when administered twice daily.
The short-term efficacy of brinzolamide 1% administered twice and three times daily was equivalent to that of three-times-daily dorzolamide 2%; mean daily IOP reduction from baseline was in the range of 13.2–21.8% for twice-daily and 13.2–21.5% for three-times-daily brinzolamide 1%, and 15.7–22.9% for dorzolamide 2% in two 3-month clinical trials in a total of 921 patients with POAG or OH (all p < 0.001). Clinically relevant reductions from baseline of the morning trough IOP were maintained long term with brinzolamide 1% throughout an 18-month trial involving 351 patients with POAG or OH (13.2% and 12.6%, respectively, in twice- and three-times-daily regimens; both p < 0.0001 vs baseline), although the effect was significantly lower than with twice-daily timolol 0.5% ophthalmic solution (20.5%; p < 0.0002).
After 3 months of treatment in two monotherapy trials, brinzolamide 1% administered twice or three times daily, respectively, produced IOP response (i.e. an IOP reduction ≥5mm Hg) or control (i.e. IOP of ≤21mm Hg) in up to 75.7% and 80.1% of patients, compared with up to 80.0% of patients who received dorzolamide 2% three times daily and up to 82.0% of patients who received timolol 0.5% twice daily.
As Adjunctive Therapy: Brinzolamide 1% was as effective as dorzolamide 2% (14.1–22.0% vs 14.0–21.2%) and superior to placebo (13.2–16.6% vs 4.4–10.4%; all p ≤ 0.03) in lowering IOP in patients with POAG or OH (n = 321) when used adjunctively with timolol 0.5% ophthalmic solution in twice- and three-times-daily regimens, respectively, at all timepoints during two 3-month trials. Furthermore, in the active-controlled trial (n = 213), a similar proportion of patients achieved IOP reduction or control with brinzolamide plus timolol and dorzolamide plus timolol (50.0–89.3% vs 43.9–85.4%, respectively).
Tolerability
Brinzolamide 1% ophthalmic suspension administered twice or three times daily was well tolerated in randomised, double-blind, multicentre, comparative, mono-therapy clinical trials in a total of 1626 patients with POAG or OH; brinzolamide-related adverse events were nonserious and generally mild to moderate in severity, occurring mostly at the time of eyedrop instillation and usually resolving without treatment.
In the only long-term (18-month) clinical trial, adverse events and inadequate IOP control were the most common reasons for treatment discontinuation and accounted, respectively, for 39% and 17% of discontinuations in the twice-, and 27% and 21% in the three-times-daily brinzolamide groups, and for 30% and 4% in the timolol group.
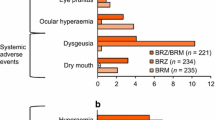
In all clinical trials, the most common ocular adverse events related to brinzolamide treatment were transient blurring of vision and ocular discomfort (i.e. stinging and burning ocular sensations). The incidence of blurred vision in 3- and 18-month trials was relatively low (3–8% with twice-daily, and 3.6–5.2% with three-times-daily regimens) and not statistically significantly greater than that reported in patients receiving three-times-daily dorzolamide 2% (0.6% and 0.8%) or placebo (1.5%), or twice-daily timolol 0.5% (0% and 5.3%). In clinical trials of up to 18 months duration, ocular discomfort at the time of instillation occurred ≈3–9 times less frequently with brinzolamide 1% than with dorzolamide 2% (p < 0.05). In addition, burning and/or stinging sensation was significantly less intense with brinzolamide 1% compared with dorzolamide 2% (p < 0.0001) in two weekly comfort studies in a total of 198 patients with POAG or OH.
Results of a prospective, nonblind, noncomparative, crossover, multicentre clinical study in 447 evaluable patients with glaucoma indicate that substituting dorzolamide 2% with brinzolamide 1% for mono- or adjunctive therapy may provide improved ocular comfort. One to 3 months after the switch, 69%, 26% and 5% of patients, respectively, reported improvement, no change, and worsening in ocular comfort. Consequently, more than twice as many patients preferred brinzolamide over dorzolamide for their glaucoma therapy (59% vs 26%; p-value not reported).
Brinzolamide did not affect visual acuity, visual fields, cup-to-disc ratio and other ocular signs, and corneal endothelial cell function (see Overview of Pharmacodynamic Properties summary) in patients with POAG or OH receiving treatment for up to 18 months during clinical trials.
Abnormal (i.e. bitter or sour) taste was the only local, nonocular adverse event associated with brinzolamide 1%, which occurred in ≥3% of patients across monotherapy and adjunctive therapy trials. The incidence of abnormal taste with brinzolamide use was dose- and frequency-related (6.8–12.1% with brinzolamide 1% three times daily), and was not statistically significantly different than that with dorzolamide 2%.
Topical brinzolamide 1% does not appear to produce acid-base or electrolyte disturbances and was not associated with clinically significant systemic adverse effects, generally observed with oral CA inhibitors, in clinical trials of up to 18 months duration.
Pharmacoeconomic Analyses
Thus far, pharmacoeconomic analyses relating to the use of brinzolamide in the treatment of POAG and OH have been limited to a cost-minimisation study and two cost analyses.
The European cost-minimisation study showed that initiating medical glaucoma therapy with brinzolamide 1% in patients with POAG or OH was associated with lower (in Italy, Spain and Portugal) or similar (in France) total direct medical costs per patient over 3 months compared with dorzolamide 2%. The analysis was conducted from the perspective of healthcare payers from each country and in the context of second-line use of topical CA inhibitors, and was based on similar week 4 responder rates between brinzolamide and dorzolamide regimens from four randomised, double-blind, comparative brinzolamide clinical trials. The cost benefit of short-term brinzolamide versus dorzolamide therapy was explained by the lower administration rate of brinzolamide (twice daily) with equal efficacy to dorzolamide (three times daily) [see Therapeutic Efficacy summary] and lower rate of therapy discontinuation with brinzolamide than with dorzolamide due to better local tolerability (see Tolerability summary).
Two US cost analyses conducted from a payer’s perspective and covering similar periods (1999 and 1998–2000) both showed that twice-daily therapy with brinzolamide 1% incurred less cost than with dorzolamide 2%. Both studies also found that glaucoma monotherapy was less costly with twice-daily brinzolamide 1% than with brimonidine 2% (twice daily) or latanoprost 0.005% (once daily), but more costly than with generic timolol maleate 0.5% (twice daily).
Dosage and Administration
Brinzolamide 1 % ophthalmic suspension is indicated for the treatment of elevated IOP in patients with POAG or OH. The recommended dosage of brinzolamide in Europe is one drop instilled in the conjunctival sac of the affected eye(s) twice daily (for use as either monotherapy or adjunctive therapy with topical ophthalmic β-blockers). In Europe, brinzolamide monotherapy is also indicated in patients unresponsive to β-blockers or in whom β-blockers are contraindicated. In the US, the recommended administration regimen for brinzolamide as monotherapy is three times daily; formal US recommendations for the use of brinzolamide in combination with other topical antiglaucoma drugs have not been made.
Brinzolamide is not recommended in the US and is contraindicated in Europe for the treatment of patients with severe renal impairment (creatinine clearance <30 mL/min), as brinzolamide and its metabolites are excreted predominantly by the kidney (see Overview of Pharmacokinetic Properties summary). In patients with hepatic impairment, either the use of brinzolamide is not recommended (US) or the drug should be used with caution (Europe). It appears prudent to avoid the use of brinzolamide in patients with previous hypersensitivity reactions to sulfonamides.
Wearers of soft contact lenses should allow a period of at least 15 minutes to elapse between instillation of brinzolamide and the re-insertion of lenses.







Similar content being viewed by others
Notes
Use of tradenames is for product identification purposes only and does not imply endorsement.
References
Kwon YH, Caprioli J. Primary open-angle glaucoma. In: Tasman W, Jaeger EA, editors. Duane’s clinical ophthalmology on CD-ROM. 2000 ed. Philadelphia (PA): Lippincott Williams & Wilkins Publishers, Inc., 2000; Clinical Volume 3; Chapter 52: 1–41
The Royal College of Ophthalmologists. Guidelines for the management of ocular hypertension and primary open angle glaucoma [online]. Available from URL: http://www.rcophth.ac.uk/publications/index.html [Accessed 2003 May 12]
Sommer A. Intraocular pressure and glaucoma. Am J Ophthalmol 1989 Feb 15; 107(2): 186–8
Weitzman M, Caprioli J. Medical Therapy of Glaucoma. In: Tasman W, Jaeger EA, editors. Duane’s clinical ophthalmology on CD-ROM. 2000 ed. Philadelphia (PA): Lippincott Williams & Wilkins Publishers, Inc., 2000; Clinical Volume 3; Chapter 56: 41–133
Kanski JJ, McAllister JA. Glaucoma: a colour manual of diagnosis and treatment. London: Butterworths & Co. (Publishers) Ltd, 1989
Nilsson SFE, Bill A. The normal anterior segment; section II. Physiology and neurophysiology of aqueous humor inflow and outflow. In: Kaufman PL, Mittag TW, editors. Glaucoma. London: Mosby-Year Book Europe Ltd., 1994; 7: 1.17–34
Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002; 120: 701–13
Kaur IP, Smitha R, Aggarwal D, et al. Acetazolamide: future perspective in topical glaucoma therapeutics. Int J Pharm 2002 Nov 6; 248(1–2): 1–14
Sugrue MF, Harris A, Adamsons I. Dorzolamide hydrochloride: a topically active, carbonic anhydrase inhibitor for the treatment of glaucoma. Drugs Today 1997 Jun; 33(5): 283–98
Alcon (USA) Inc. Azopt® (brinzolamide ophthalmic suspension) 1%. Prescribing information [online]. Available from URL: http://www.alconlabs.com/us/aj/products/RxTher/azopt-pi.jhtml [Accessed 2003 Jun 17]
Alcon Laboratories (UK) Ltd. Azopt (brinzolamide eye drops suspension) 1%. Summary of product characteristics. EMEA [online]. Available from URL: http://www.eudra.org/humandocs/Humans/EPAR/Azopt/Azopt.htm [Accessed 2003 Apr 17]
Hall R, Havner G, Baker J, et al. Brinzolamide. Analytical Profiles of Drug Substances and Excipients 1999; 26: 47–96
Dean T, May J, Chen HH, et al. Brinzolamide (AL-4862) suspension is a new topically active carbonic anhydrase inhibitor in the Dutch-belted rabbit and cynomolgus monkey [abstract no. 3786-B387]. Invest Ophthalmol Vis Sci 1997 Mar 15; 38(4): S813
DeSantis L. Preclinical overview of brinzolamide. Surv Ophthalmol 2000 Jan; 44Suppl. 2: S119–29
Merck & Co., Inc. Trusopt® (dorzolamide hydrochloride ophthalmic solution) Sterile Ophthalmic Solution 2% [prescribing information]. West Point (PA), USA, 1999
Maren TH. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev 1967 Oct; 47(4): 595–781
Sugrue MF. Pharmacological and ocular hypotensive properties of topical carbonic anhydrase inhibitors. Prog Retin Eye Res 2000 Jan; 19(1): 87–112
March WF, Ochsner KI. The long-term safety and efficacy of brinzolamide 1.0% (Azopt) in patients with primary open-angle glaucoma or ocular hypertension. Brinzolamide Long-Term Therapy Study Group. Am J Ophthalmol 2000 Feb; 129(2): 136–43
Wistrand PJ, Garg LC. Evidence of a high-activity C type of carbonic anhydrase in human ciliary processes. Invest Ophthalmol Vis Sci 1979 Aug; 18(8): 802–6
Ingram CJ, Brubaker RF. Effect of brinzolamide and dorzolamide on aqueous humor flow in human eyes. Am J Ophthalmol 1999 Sep; 128(3): 292–6
Silver LH. Dose-response evaluation of the ocular hypotensive effect of brinzolamide ophthalmic suspension (Azopt). Brinzolamide Dose-Response Study Group. Surv Ophthalmol 2000 Jan; 44Suppl. 2: S147–53
Sall K. The efficacy and safety of brinzolamide 1 % ophthalmic suspension (Azopt) as a primary therapy in patients with open-angle glaucoma or ocular hypertension. Brinzolamide Primary Therapy Study Group. Surv Ophthalmol 2000 Jan; 44Suppl. 2: S155–62
Silver LH. Clinical efficacy and safety of brinzolamide (Azopt), a new topical carbonic anhydrase inhibitor for primary open-angle glaucoma and ocular hypertension. Brinzolamide Primary Therapy Study Group. Am J Ophthalmol 1998 Sep; 126(3): 400–8
Shin D. Adjunctive therapy with brinzolamide 1% ophthalmic suspension (Azopt) in patients with open-angle glaucoma or ocular hypertension maintained on timolol therapy. Brinzolamide Adjunctive Therapy Study Group. Surv Ophthalmol 2000 Jan; 44Suppl. 2: S163–8
Michaud J-E, Friren B. Comparison of topical brinzolamide 1 % and dorzolamide 2% eye drops given twice daily in addition to timolol 0.5% in patients with primary open-angle glaucoma or ocular hypertension. International Brinzolamide Adjunctive Study Group. Am J Ophthalmol 2001 Aug; 132(2): 235–43
Konowal A, Morrison JC, Brown SVL, et al. Irreversible corneal decompensation in patients treated with topical dorzolamide. Am J Ophthalmol 1999 Apr; 127(4): 403–6
Adamson I. Irreversible corneal decompensation in patients treated with topical dorzolamide [letter]. Am J Ophthalmol 1999 Dec; 128(6): 774–5
Flammer J. The vascular concept of glaucoma. Surv Ophthalmol 1994; 38: 3–6
Hitchings RA. Intraocular pressure and circulation at the disc in glaucoma. Acta Ophthalmol Scand 1997; 75 (220 Suppl.): 15–22
Buckley MM-T, Goa KL, Clissold SP. Ocular betaxolol: a review of its pharmacological properties, and therapeutic efficacy in glaucoma and ocular hypertension. Drugs 1990 Jul; 40(1): 75–90
Harris A, Anderson DR, Pillunat L, et al. Laser Doppler flowmetry measurement of changes in human optic nerve head blood flow in response to blood gas perturbations. J Glaucoma 1996; 5(4): 258–65
Alm A, Bill A. The oxygen supply to the retina, II. Effects of high intraocular pressure and of increased arterial carbon dioxide tension on uveal and retinal blood flow in cats. Acta Physiol Scand 1972; 84: 306–19
Riva CE, Shonat RD, Petrig BL, et al. Noninvasive measurement of the optic nerve head circulation. In: Lambrou GN, Greve EL, editors. Ocular blood flow in glaucoma. Amsterdam, Berkeley, Milano: Kugler & Ghedini Publications, 1989
Kontos HA, Wei EP, Raper AJ, et al. Local mechanism of CO2 action on cat pial arterioles. Stroke Mar-Apr 1977; 8(2): 226–9
Sampaolesi J, Tosi J, Darchuk V, et al. Antiglaucomatous drugs effects on optic nerve head flow: design, baseline and preliminary report. Int Ophthalmol 2001; 23(4–6): 359–67
Rouland J-F, Le Pen C, Gouveia Pinto C, et al. Cost-minimisation study of dorzolamide versus brinzolamide in the treatment of ocular hypertension and primary open-angle glaucoma: in four European countries. Pharmacoeconomics 2003; 21(3): 201–13
Silver LH. Ocular comfort of brinzolamide 1.0% ophthalmic suspension compared with dorzolamide 2.0% ophthalmic solution: results from two multicenter comfort studies. Brinzolamide Comfort Study Group. Surv Ophthalmol 2000 Jan; 44Suppl. 2: S141–5
Barnebey H, Kwok SY. Patients’ acceptance of a switch from dorzolamide to brinzolamide for the treatment of glaucoma in a clinical practice setting. Clin Ther 2000 Oct; 22(10): 1204–12
Clark D. Hawthorne effect [online]. Available from URL: http://www.nwlink.com/~donclark/hrd/history/hawthorne.html [Accessed 2003 Mar 28]
Vogel R, Strahlman E, Rittenhouse KD. Adverse events associated with commonly used glaucoma drugs. Int Ophthalmol Clin 1999; 39(2): 107–24
Fiscella RG, Geller JL, Gryz LL, et al. Cost considerations of medical therapy for glaucoma. Am J Ophthalmol 1999 Oct; 128(4): 426–33
Vold SD, Riggs WL, Jackimiec J. Cost analysis of glaucoma medications: a 3-year review. J Glaucoma 2002 Aug; 11(4): 354–8
Halpern MT, Sorensen S, Covert D, et al. Longitudinal glaucoma treatment patterns with brinzolamide versus dorzolamide. J Med Econ 2000; 3: 111–20
WHO Information Fact Sheets. Blindness and visual disability part II of VII: major causes worldwide (Fact Sheet N143) [online]. Available from URL: http://www.who.int/inf-fs/en/factl43.html [Accessed 2003 May 2]
American Academy of Ophthalmology Preferred Practice Patterns Committee Glaucoma Panel. Preferred practice pattern™: primary open-angle glaucoma. San Francisco (CA): American Academy of Ophthalmology, 2000
The World Health Report, 1998. Causes of blindness, 1997 worldwide [online]. Available from URL: http://www.who.int/pbd/pbl/data/blindness_world_prevalence_1997.pdf [Accessed 2003 May 12]
Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol 1996 May; 80(5): 389–93
Schwartz M, Belkin M, Yoles E, et al. Potential treatment modalities for glaucomatous neuropathy: neuroprotection and neuroregeneration. J Glaucoma 1996; 5(6): 427–32
Caprioli J. Neuroprotection of the optic nerve in glaucoma. Acta Ophthalmol Scand 1997; 75(4): 364–7
Sugrue MF. New approaches to antiglaucoma therapy. J Med Chem 1997 Aug 29; 40(18): 2793–809
McKinnon SJ. Glaucoma, apoptosis, and neuroprotection. Curr Opin Ophthalmol 1997; 8(II): 28–37
Yoles E, Schwartz M. Potential neuroprotective therapy for glaucomatous optic neuropathy. Surv Ophthalmol Jan-Feb 1998; 42(4): 367–72
Chung HS, Harris A, Evans DW, et al. Vascular aspects in the pathophysiology of glaucomatous optic neuropathy. Surv Ophthalmol 1999 Jun; 43Suppl. 1: S43–50
Heijl A, Laske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002; 120: 1268–79
Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol 2003 Jan; 121(1): 48–56
Collaborative normal-tension glaucoma study group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol 1998 Oct; 126(4): 498–505
The AGIS investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 2000 Oct; 130(4): 429–40
Lee P. Use of guidelines in the management of patients with glaucoma. Dis Manage Health Outcomes 1999 Apr; 5: 187–95
Perry CM, McGavin JK, Culy CR, et al. Latanoprost: an update of its use in glaucoma and ocular hypertension. Drugs Aging 2003; 20(6)
van Buskirk EM. Adverse reactions from timolol administration. Ophthalmology 1980 May; 87(5): 447–50
Adkins JC, Balfour JA. Brimonidine: a review of its pharmacological properties and clinical potential in the management of open-angle glaucoma and ocular hypertension. Drugs Aging 1998 Mar; 12(3): 225–41
Rait JL. Systemic effects of topical ophthalmic β-adrenoceptor antagonists. Aust N Z J Ophthalmol 1999 Feb; 27: 57–64
Hoyng PFJ, van Beek LM. Pharmacological therapy for glaucoma: a review. Drugs 2000; 59(3): 411–34
Hoyng PFJ, van Beek LM PFJ. Pharmacological therapy for glaucoma: a review. Erratum. Drugs 2002; 62(9): 1314
Harris LS, Galin MA. Dose response analysis of pilocarpineinduced ocular hypotension. Arch Ophthal 1970 Nov; 84: 605–8
Jay JL, Allan D. The benefit of early trabeculectomy versus conventional management in primary open angle glaucoma relative to severity of disease. Eye 1989; 3 (Pt 5): 528–35
Migdal C, Gregory W, Hitchings R. Long-term functional outcome after early surgery compared with laser and medicine in open-angle glaucoma. Ophthalmology 1994 Oct; 101(10): 1651–7
Glaucoma Laser Trial Research Group. The Glaucoma Laser Trial (GLT) and Glaucoma Laser Trial Follow-up Study: 7. Results. Am J Ophthalmol 1995 Dec; 120(6): 718–31
Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001 Nov; 108(11): 1943–53
Janz NK, Wren PA, Lichter PR, et al. The Collaborative Initial Glaucoma Treatment Study: interim quality of life findings after initial medical or surgical treatment of glaucoma. Ophthalmology 2001 Nov; 108(11): 1954–65
Pharmacia Corporation. FDA grants marketing approval to Xalatan® for initial treatment of elevated eye pressure [online]. Available from URL: http://www.xalatan.com/press_media/release06.htm [Accessed 2003 Aug 15]
Camras CB, Toris CB, Tamesis RR. Efficacy and adverse effects of medications used in the treatment of glaucoma. Drugs Aging 1999; 15(5): 377–88
Balfour JA, Adkins JC. Management of primary open-angle glaucoma and ocular hypertension: the potential role of latanoprost. Dis Manage Health Outcomes 1998; 4(2): 101–12
Waugh J, Jarvis B. Travoprost. Drugs Aging 2002; 19(6): 465–71; discussion 472–3
Easthope SE, Perry CM. Topical bimatoprost: a review of its use in open-angle glaucoma and ocular hypertension. Drugs Aging 2002; 19(3): 231–48
Konstas AGP, Stewart WC, Topouzis F, et al. Brimonidine 0.2% given two or three times daily versus timolol maleate 0.5% in primary open-angle glaucoma. Am J Ophthalmol 2001 Jun; 131(6): 729–33
Balfour JA, Wilde MI. Dorzolamide: a review of its pharmacology and therapeutic potential in the management of glaucoma and ocular hypertension. Drugs Aging 1997 May; 10(5): 384–403
Doyle JW, Smith MF, Tierney Jr JW. Glaucoma medical treatment-2002: does yearly cost now equal the year? Optom Vis Sci 2002; 79(8): 489–92
Rossetti L, Marchetti I, Orzalesi N, et al. Randomized clinical trials on medical treatment of glaucoma: are they appropriate to guide clinical practice? Arch Ophthalmol 1993 Jan; 111: 96–103
Ormrod D, McClellan K. Topical dorzolamide 2%/timolol 0.5%: a review of its use in the treatment of open-angle glaucoma. Drugs Aging 2000 Dec; 17(6): 477–96
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cvetkovic, R.S., Perry, C.M. Brinzolamide. Drugs Aging 20, 919–947 (2003). https://doi.org/10.2165/00002512-200320120-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002512-200320120-00008