Abstract
Introduction
Fixed-combination intraocular pressure (IOP)—lowering medications simplify treatment regimens for patients requiring 2 ocular hypotensive agents to maintain sufficiently low IOP. The aim of this study was to evaluate the safety and efficacy of fixed-combination brinzolamide 1%/brimonidine 0.2% (BBFC) versus concomitant administration of brinzolamide 1% plus brimonidine 0.2% (BRINZ + BRIM) in patients with open-angle glaucoma or ocular hypertension.
Methods
This was a prospective, phase 3, multicenter, double-masked, 6-month trial. Patients who had insufficient IOP control with monotherapy or who were receiving 2 IOP-lowering medications were randomized 1:1 to receive twice-daily BBFC or BRINZ + BRIM. IOP was assessed at 9 a.m. and 11 a.m. during week 2, week 6, month 3, and month 6 visits. The primary efficacy endpoint was mean diurnal IOP change from baseline to month 3; noninferiority was concluded if the upper limit of the 95% CI of the between-group difference was <1.5 mmHg. Supportive endpoints included mean IOP, IOP change from baseline, and percentage of patients with IOP <18 mmHg. Adverse events were recorded.
Results
The mean diurnal IOP change from baseline with BBFC (least squares mean ± standard error −8.5 ± 0.16 mmHg) was noninferior to that with BRINZ + BRIM (–8.3 ± 0.16 mmHg; mean difference −0.1 mmHg; 95% CI −0.5 to 0.2 mmHg). The upper limits of the 95% CIs were <1.5 mmHg at all time points. Decreases from baseline >8 mmHg were observed for least squares mean diurnal IOP in both groups as early as week 2 and continued to the end of the study. The results of all other supportive endpoints were similar to the primary efficacy endpoint. The most common ocular adverse drug reactions were hyperemia of the eye (reported as ocular or conjunctival hyperemia), visual disturbances, ocular allergic reactions, and ocular discomfort. Common systemic adverse drug reactions included dysgeusia, oral dryness, and fatigue/drowsiness.
Conclusion
Brinzolamide 1%/brimonidine 0.2% fixed combination was as well tolerated and effective as concomitant therapy with its components. BBFC reduces treatment burden in patients who require multiple IOP-lowering medications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glaucoma is associated with significant disability and global burden. It is without question the most common cause of irreversible blindness globally and is second only to cataracts as the most common cause of blindness overall [1]. Increased intraocular pressure (IOP) is a risk factor for worsening glaucoma-related neuropathy [2–5]; therefore, the primary treatment goal is to achieve an IOP within an acceptable target range [4]. First-line therapies for IOP reduction in developed countries include prostaglandin analogs and β-blockers; other treatment options include α2-adrenergic agonists (e.g., brimonidine), carbonic anhydrase inhibitors (e.g., brinzolamide), and parasympathomimetics [4]. Monotherapy is often insufficient to achieve target IOP; thus, combination therapy may be required [4, 6]. In addition to robust IOP-lowering efficacy, fixed-combination therapies provide multiple benefits versus treatment with the corresponding separate medications [7]. These include potentially lower cost, simplified treatment regimens, improved treatment compliance, reduced risk of drug washout, and decreased risk of corneal and ocular surface damage associated with cumulative exposure to preservatives [8–12]. For example, the preservative benzalkonium chloride is used in many topical glaucoma medications, but some studies suggest that it may be associated with corneal and conjunctival cell damage and inflammation [13, 14], tear film disruption [15], and symptoms of ocular surface disease [16] following chronic exposure. Fixed-combination glaucoma medications reduce overall preservative burden; however, all currently available fixed-combination formulations contain a β-blocker and, therefore, may be contraindicated in patients with certain medical conditions [17, 18].
A fixed combination of the carbonic anhydrase inhibitor brinzolamide 1% and the α2-adrenergic agonist brimonidine 0.2% (BBFC; Simbrinza®, Alcon Laboratories, Inc., Fort Worth, TX, USA) was approved in the United States in April 2013. BBFC, which is indicated for the reduction of elevated IOP in patients with primary open-angle glaucoma or ocular hypertension, exerts its IOP-lowering efficacy via two complementary mechanisms: decreased aqueous production by brimonidine and brinzolamide and increased aqueous outflow with brimonidine [4]. In clinical trials, BBFC administered three times daily (TID; a dosing regimen consistent with the approved dosing regimens of brinzolamide and brimonidine in the United States) was more effective in lowering IOP than brinzolamide or brimonidine as monotherapy, and BBFC had a safety profile similar to that of the individual components [12, 19–21]. A twice-daily (BID) dosing regimen is approved for brinzolamide and brimonidine in most countries in the European Union. A recent multinational, randomized, double-masked clinical trial of 560 patients demonstrated significantly greater IOP-lowering efficacy of BBFC administered BID versus monotherapy with either of its components after 6 months of treatment [22]. The relative IOP-lowering efficacy of BID BBFC compared with concomitant therapy with its unfixed components remains to be demonstrated.
The aim of the present study was to evaluate the safety and IOP-lowering efficacy of BBFC administered BID versus concomitant administration of the unfixed combination of brinzolamide 1% and brimonidine 0.2% (BRINZ + BRIM) in patients with open-angle glaucoma or ocular hypertension.
Methods
Study Design and Intervention
This was a phase 3, randomized, multicenter, multinational, double-masked, parallel-group, noninferiority trial conducted at 102 sites in Europe, Central America, South America, Australia, New Zealand, India, Canada, and the United States from May 2011 to January 2013. The study evaluated the safety and efficacy of BID BBFC compared with BID BRINZ + BRIM in reducing IOP in patients with open-angle glaucoma or ocular hypertension who, in the medical opinion of the investigator examining the patient, were insufficiently controlled on monotherapy or who were receiving multiple IOP-lowering medications (ClinicalTrials.gov identifier: NCT01309204). The study was approved by Comitato Etico Unico Per la Provincia di Parma and Comitati Etico Direzione Scientifica Fondazione IRCCS (SAG), Research Ethics Committee for Wales (JL), Comite Independiente de Etica para Ensayos en Farmacologia Clinica (ACS), and Ethik-Kommission-der Bayerischen Landesärztekammer (TH). This study was compliant with the Health Insurance Portability and Accountability Act (HIPAA) and the ethical standards set forth by the Declaration of Helsinki and Good Clinical Practice. All patients provided written informed consent before study initiation.
During the screening visit, patients reported their medical histories and concomitant medications, were evaluated against inclusion and exclusion criteria, and received a medical examination (e.g., IOP measurements). After an appropriate washout period (as previously reported [19, 20]), patients confirmed discontinuation of ocular hypotensive agents and underwent bilateral IOP measurement at 9 a.m. and 11 a.m. at 2 eligibility visits. Patients were eligible for inclusion if mean IOP measurements for ≥1 eye (the same eye) were 24–36 mmHg at 9 a.m. and 21–36 mmHg at 11 a.m. during both eligibility visits; mean IOP could not exceed 36 mmHg in either eye at any time during the study. Time-matched IOP measurements from each eligibility visit were averaged to calculate the baseline IOP at each time point.
Eligible patients were randomly assigned 1:1 using an interactive web response system to receive BBFC or BRINZ + BRIM for 6 months. Randomization was determined using a block design, stratified by study center and mean 9 a.m. baseline IOP (24−27 or 28−36 mmHg). Because the study medications differed in appearance (suspension vs solution), all patients were administered doses from 2 bottles with identical labels. Patients self-administered either BBFC and vehicle (1 drop each) or BRINZ + BRIM (1 drop each brinzolamide 1% and brimonidine 0.2%) at 9 a.m. ± 30 min and 9 p.m. ± 30 min in both eyes; during study visits, designated study personnel administered the 9 a.m. dose after IOP was measured. Drops were administered ≥10 min apart to avoid washout effects.
Patients
Complete patient inclusion and exclusion criteria are provided in Table 1.
Outcomes
The primary efficacy endpoint was the mean change from baseline to month 3 in diurnal IOP, which was calculated as the average of the 9 a.m. and 11 a.m. time points. The 9 a.m. and 11 a.m. time points were selected because they correspond approximately to trough (12 h postinstillation) and peak (+2 h postinstillation) IOP-lowering efficacy of brinzolamide and brimonidine [23]. At trough (9 a.m.) and peak (+2 h; 11 a.m.), IOP was expected to be at the highest and lowest points, respectively, on the diurnal curve for most patients. Supportive efficacy endpoints included:
-
Mean diurnal IOP change from baseline to week 2, week 6, and month 6;
-
Mean IOP at each study visit and time point (i.e., week 2, week 6, month 3, and month 6 at 9 a.m. and 11 a.m.);
-
Mean IOP change from baseline at each study visit and time point;
-
Mean IOP percentage change from baseline at each study visit and time point;
-
Percentage of patients with IOP <18 mmHg at each study visit and time point.
Intraocular pressure was measured by a tonometer operator and a tonometer reader; patient treatment was masked to the operator and reader throughout the study. At each time point (i.e., 9 a.m. and 11 a.m.) of each treatment study visit, at least two consecutive measures of IOP were obtained for each eye using a Goldmann applanation tonometer. If the initial two measurements differed by >4 mmHg, a third measurement was taken and the two most similar measurements averaged; if all measurements differed by similar amounts, all three were averaged. One eye from each patient was designated the “study eye,” and data from only this eye were included in the efficacy analyses. Among patients who dosed only one eye during the study, the dosed eye was selected as the study eye. If both eyes were dosed during the study, the worse evaluable eye (defined as the eye with the higher IOP at 9 a.m. averaged across the two eligibility visits) was selected as the study eye. If IOP was equal in both eyes, the eye with the higher IOP at 11 a.m. (averaged across two eligibility visits) was designated as the study eye; if IOP measurements were equal in both eyes at 11 a.m., the right eye was selected.
Safety outcomes included adverse event (AE) reporting, best-corrected visual acuity (BCVA) corneal thickness, visual field function, slit-lamp biomicroscopy (cornea, iris/anterior chamber, lens, eyelids), dilated fundus examination (vitreous, retina/macula/choroid, optic nerve including cup-to-disc ratio), and cardiovascular assessment (pulse, blood pressure). AE data were collected at all on-therapy study visits. BCVA and cardiovascular assessments were performed at screening and at all postscreening visits at 9 a.m. before instillation of study medication. Assessments of corneal thickness, visual field function, and slit-lamp biomicroscopy and dilated fundus examination were performed at screening and at 11 a.m. during the month 6 visit after assessment of IOP. AEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA), version 13.0, and are presented as MedDRA Preferred Terms.
Statistical Analyses
Demographic parameters and baseline characteristics were summarized using descriptive statistics. In the per-protocol (PP) population (i.e., all patients meeting pre-randomization inclusion and exclusion criteria who received study drug and completed ≥1 on-therapy study visit), comparisons of between-group differences for the primary endpoint were based on least squares (LS) means derived from a statistical model that accounted for correlated intrapatient IOP measurements, study center, and baseline IOP and were made using two-sided t tests. Similar methodology was used to assess mean diurnal change in IOP from baseline at week 2, week 6, and month 6; however, the P values for these supportive endpoints were considered descriptive in nature. Descriptive statistics are provided for all other supportive efficacy endpoints. All primary and supportive endpoints were also evaluated within the intent-to-treat (ITT) population (i.e., all patients who received study drug and completed ≥1 on-therapy study visit) to supplement the PP analysis.
For the noninferiority test, two-sided 95% CIs were constructed for the between-group differences in mean change from baseline in diurnal IOP at each visit and time point on an observed-case basis in the PP population. Noninferiority of BBFC to BRINZ + BRIM was established if the upper limit of the 95% CI of the between-group difference in mean change from baseline to month 3 in diurnal IOP was <1.5 mmHg. A Cochran–Mantel–Haenszel test was used to analyze between-group differences for categorical parameters across study center and baseline IOP strata. Safety data were examined in all patients who were exposed to study drug and were summarized using descriptive statistics. All analyses were performed using SAS® software (SAS Institute, Cary, NC, USA).
A total of approximately 820 patients were planned to be enrolled. With 340 patients per group in the primary efficacy analysis, the study would have at least 90% power that a two-sided 95% CI of the difference in mean IOP between the treatment groups at month 3 would be within ±1.5 mmHg. Because only one side of this tolerance region was relevant for the noninferiority comparison, the upper limit of the CI was compared with 1.5 mmHg. Thus, approximately 410 patients per treatment group were planned for enrollment to account for patient dropout or loss to follow-up.
Results
Patients
In total, 1,190 patients were enrolled and 890 were randomly allocated to treatment with BBFC (n = 451) or BRINZ + BRIM (n = 439; Fig. 1). Among patients randomized to treatment, 8.1% reported pre-study use of BRIM, 3.6% reported use of BRIM/timolol fixed combination, and 0.2% reported use of BRIM/timolol/dorzolamide triple fixed combination; the majority of patients reported using ≤2 ocular hypotensive agents. The study was completed by 83.8% of enrolled patients. Two patients were randomized to receive BRINZ + BRIM but were administered BBFC because of drug misallocations; these patients were included in the BRINZ + BRIM group for efficacy analyses and in the BBFC group for safety analyses. A similar percentage of patients in each treatment group completed the study (BBFC 85.4%; BRINZ + BRIM 82.2%). Demographics and baseline disease characteristics, including baseline IOP, were similar between the treatment groups (Table 2); most patients were women, white, and diagnosed with open-angle glaucoma.
Patient disposition. Percentages reflect number of patients randomized to treatment for each group. Asterisk indicates patients analyzed according to treatment received; two patients were randomized to receive BRINZ + BRIM but actually received BBFC. AE adverse event, BBFC brinzolamide 1%/brimonidine 0.2% fixed combination, BRINZ + BRIM concomitant unfixed brinzolamide 1% and brimonidine 0.2%, IOP intraocular pressure, ITT intent-to-treat, PP per-protocol
Efficacy
In the PP population, LS mean ± standard error (SE) diurnal IOP change from baseline at month 3 was –8.5 ± 0.16 mmHg for patients receiving BBFC and –8.3 ± 0.16 mmHg for patients receiving BRINZ + BRIM (mean between-group difference −0.1 mmHg; 95% CI −0.5 to 0.2 mmHg; Fig. 2). Because the upper limit of the 95% CI of the between-group difference was less than the prespecified margin of 1.5 mmHg, BBFC was demonstrated to be noninferior to BRINZ + BRIM. Similar results were obtained within the ITT population (data not shown).
LS mean changes in diurnal IOP (i.e., average of IOP at 9 a.m. and 11 a.m.) from baseline (per-protocol population). Error bars represent standard errors. LS mean between-group differences and 95% CIs for the primary efficacy endpoint (month 3) and supportive efficacy endpoints (week 2 and month 6) are provided. BBFC brinzolamide 1%/brimonidine 0.2% fixed combination, BRINZ + BRIM concomitant unfixed brinzolamide 1% and brimonidine 0.2%, IOP intraocular pressure, LS least squares
Mean and percentage change in IOP from baseline at each study visit and time point were similar between treatment groups (Table 3). Decreases from baseline of over 8 mmHg were observed for LS mean diurnal IOP in both groups as early as week 2 and continued to the end of the study (Fig. 2). The upper limits of the 95% CIs at all time points were <1.5 mmHg; therefore, BBFC was noninferior to BRINZ + BRIM throughout the study.
At all study visits and time points, mean IOP was similar in the BBFC and BRINZ + BRIM groups and ranged from 19.1 to 19.7 mmHg at 9 a.m. measurements and from 16.0 to 16.5 mmHg at 11 a.m. measurements (Table 3).
The percentage of patients achieving IOP <18 mmHg was also similar with both treatments; at the time of peak morning efficacy (11 a.m.), the percentage of patients with IOP <18 mmHg across study visits was 68.9–71.6% for those receiving BBFC and 65.8–71.6% for those receiving BRINZ + BRIM. Week 6 IOP data (not shown) were similar to efficacy data at other study visits.
Safety
The safety profile of BBFC was consistent with the known safety profiles of its individual components, and BBFC did not result in additional risk to patients.
A similar percentage of patients receiving BBFC or BRINZ + BRIM experienced serious AEs (SAEs; i.e., approximately 2% in both treatment groups; Table 4). One patient who received BRINZ + BRIM died of a myocardial infarction that was assessed as unrelated to study drug. The majority of SAEs were reported as single events, resolved over the course of the study, and did not interrupt use of the study drug. No patterns emerged to suggest a patient-safety issue with BBFC, and of all SAEs, only corneal erosion was considered related to study drug. All other reported SAEs were assessed by the study investigator as unrelated to the use of study medication.
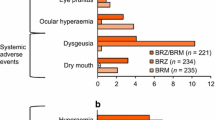
Adverse drug reactions (ADRs; i.e., AEs assessed as related to treatment) were reported in a similar percentage of patients receiving BBFC (23.5%) and BRINZ + BRIM (26.8%; Table 4). The majority of ADRs reported during the study were local ocular side effects with a known causal association with the individual components. The most common ocular ADRs were hyperemia of the eye (reported as ocular or conjunctival hyperemia), visual disturbances, ocular allergic type reactions, and ocular discomfort. Common systemic ADRs reported in the study included dysgeusia, oral dryness, and fatigue/drowsiness.
Study treatment was discontinued because of an AE in 13.3% of patients in the BRINZ + BRIM group and 10.6% of patients in the BBFC group; discontinuations because of a treatment-related nonserious AE were reported for 11.7% and 10.0% of patients receiving BRINZ + BRIM and BBFC, respectively (Table 4). The majority of discontinuations in both groups were attributable to local ocular events associated with the use of the individual components (e.g., ocular discomfort, hyperemia of the eye, ocular allergies). No clinically meaningful alterations in other ocular or cardiovascular assessments were observed.
Discussion
In this randomized phase 3 trial, change in LS mean ± SE diurnal IOP from baseline was similar with BBFC (–8.5 ± 0.16 mmHg) and BRINZ + BRIM (–8.3 ± 0.16 mmHg; LS mean between-group difference −0.1 mmHg; 95% CI −0.5 to 0.2 mmHg) after 3 months, and the criterion for noninferiority was met. The safety profile of BBFC was consistent with the known safety profiles of the individual components administered concomitantly; no new AEs were observed.
Intraocular pressure reduction was previously demonstrated to be significantly greater with BBFC administered BID compared with either BRINZ or BRIM monotherapy administered BID [22]. In the current study, the IOP-lowering efficacy of BID BBFC was similar to that of BID BRINZ + BRIM. Furthermore, IOP reduction achieved with BBFC administered BID (mean percentage IOP reductions from baseline at 3 months, 28.6–37.6%) was similar to that previously observed with BBFC administered TID over similar morning time points at 3 months (approximately 24–34%) [19]. Longer term IOP reductions at 6 months were also similar with BID versus TID dosing of BBFC [12].
The safety of BBFC was consistent with BRINZ + BRIM and the known safety profiles of the individual components and did not result in additional risk to patients. The incidence of SAEs with BBFC administered BID (2.4%) was similar to that previously reported for this dosing regimen in a similar patient population (2.6%) [22]. The safety profile for BBFC was consistent between the 2 studies; the most common BBFC-related ADR in both trials was hyperemia [22]. The rate of ADRs in the current study after 6 months of BID treatment with BBFC (23.5%) was similar to that of BRINZ + BRIM (26.8%) and was lower than the rate reported with BBFC administered TID at 6 months (33.0%) [12].
Fixed-combination medications have several advantages over concomitant dosing of two separate medications, including simplified dosing (1 bottle versus 2) [9], increased tolerability and reduction of ocular symptoms through reduced cumulative exposure to preservatives [24], reduced cost [25, 26], and elimination of potential washout effects associated with instillation of multiple concomitant drops [8]. In addition, cumulative exposure to the components of glaucoma medications may lead to corneal and conjunctival alterations and ocular surface damage [10, 11]. Preservatives (e.g., benzalkonium chloride) in glaucoma medications are associated with ocular surface toxicity and damage that decrease patients’ quality of life [11, 27, 28]. Active compounds in glaucoma medications may contribute to inflammation, and epithelial modifications have been observed by laser scanning confocal microscopy in patients receiving more than two medications [11]. However, most side effects of glaucoma medications are thought to be caused by nonactive components such as preservatives and excipients. Fixed combinations such as BBFC reduce cumulative exposure to nonactive agents in glaucoma medications by reducing the number of daily instillations. Furthermore, BBFC contains considerably less benzalkonium chloride (0.03 mg/mL) compared with concomitant use of its currently marketed components [BRINZ (Azopt®; Alcon), 0.15 mg/mL; BRIM (Alphagan®; Allergan Inc., Irvine, CA, USA), 0.05 mg/mL] and may therefore reduce the risk of ocular surface damage, medication intolerability, and associated noncompliance. Up to 80% of patients are noncompliant with their prescribed IOP-lowering therapies, and noncompliance is increased in regimens requiring >2 doses per day [29]. By minimizing the reasons for noncompliance (e.g., dose complexity, intolerability) [9], BBFC may increase adherence, thereby improving overall IOP reduction. Previous reports have demonstrated that topical β-blockers were contraindicated for as many as 60% of glaucoma patients receiving these medications to manage their IOP [30, 31]. As the only fixed combination glaucoma therapy currently available that does not contain a β-blocker, BBFC may be particularly useful for those patients for whom a β-blocker such as timolol is contraindicated.
A limitation of this clinical study was that it aimed to demonstrate noninferior efficacy and safety of a fixed combination versus concomitant administration of the same individual medications, which prevented direct comparison with other classes of IOP-lowering medications (e.g., latanoprost/timolol combinations); therefore, subsequent trials will be required to address this comparison. Also, IOP was not assessed throughout a full diurnal period and included only two time points, which prevented comparison with previous TID dosing studies at other daily time periods. Future studies evaluating the noninferiority of BBFC compared with its unfixed components in additional patient populations (e.g., patients with normal tension glaucoma) will provide additional valuable information about the efficacy of BBFC.
Conclusions
In conclusion, BBFC BID was noninferior to BRINZ + BRIM BID for reducing elevated IOP in patients with open-angle glaucoma or ocular hypertension, and BBFC was not associated with any additional safety risks to patients relative to the known risks of the individual components. Thus, BBFC may be a useful treatment option for patients who require effective IOP lowering, for those with inadequate response to brinzolamide or brimonidine monotherapy, or for patients in whom β-blockers or prostaglandin analogs are contraindicated.
References
Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–8.
Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res. 2012;31:702–19.
Calkins DJ, Horner PJ. The cell and molecular biology of glaucoma: axonopathy and the brain. Invest Ophthalmol Vis Sci. 2012;53:2482–4.
European Glaucoma Society. Terminology and Guidelines for Glaucoma. 3rd ed. Savona: Editrice Dogma S.r.l; 2008.
Nickells RW, Howell GR, Soto I, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci. 2012;35:153–79.
Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13.
Hollo G, Topouzis F, Fechtner RD. Fixed-combination intraocular pressure-lowering therapy for glaucoma and ocular hypertension: advantages in clinical practice. Expert Opin Pharmacother. 2014;15:1737–47.
Chrai SS, Makoid MC, Eriksen SP, Robinson JR. Drop size and initial dosing frequency problems of topically applied ophthalmic drugs. J Pharm Sci. 1974;63:333–8.
Higginbotham EJ. Considerations in glaucoma therapy: fixed combinations versus their component medications. Clin Ophthalmol. 2010;4:1–9.
Mastropasqua L, Agnifili L, Mastropasqua R, Fasanella V. Conjunctival modifications induced by medical and surgical therapies in patients with glaucoma. Curr Opin Pharmacol. 2013;13:56–64.
Mastropasqua L, Agnifili L, Mastropasqua R, et al. In vivo laser scanning confocal microscopy of the ocular surface in glaucoma. Microsc Microanal. 2014;20:879–94.
Whitson JT, Realini T, Nguyen QH, McMenemy MG, Goode SM. Six-month results from a phase III randomized trial of fixed-combination brinzolamide 1% + brimonidine 0.2% versus brinzolamide or brimonidine monotherapy in glaucoma or ocular hypertension. Clin Ophthalmol. 2013;7:1053–60.
Goto Y, Ibaraki N, Miyake K. Human lens epithelial cell damage and stimulation of their secretion of chemical mediators by benzalkonium chloride rather than latanoprost and timolol. Arch Ophthalmol. 2003;121:835–9.
Noecker RJ, Herrygers LA, Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004;23:490–6.
Januleviciene I, Derkac I, Grybauskiene L, Paulauskaite R, Gromnickaite R, Kuzmiene L. Effects of preservative-free tafluprost on tear film osmolarity, tolerability, and intraocular pressure in previously treated patients with open-angle glaucoma. Clin Ophthalmol. 2012;6:103–9.
Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86:418–23.
Distelhorst JS, Hughes GM. Open-angle glaucoma. Am Fam Physician. 2003;67:1937–44.
Gandolfi SA, Chetta A, Cimino L, Mora P, Sangermani C, Tardini MG. Bronchial reactivity in healthy individuals undergoing long-term topical treatment with beta-blockers. Arch Ophthalmol. 2005;123:35–8.
Katz G, Dubiner H, Samples J, Vold S, Sall K. Three-month randomized trial of fixed-combination brinzolamide, 1%, and brimonidine, 0.2%. JAMA Ophthalmol. 2013;131:724–30.
Nguyen QH, McMenemy MG, Realini T, Whitson JT, Goode SM. Phase 3 randomized 3-month trial with an ongoing 3-month safety extension of fixed-combination brinzolamide 1%/brimonidine 0.2%. J Ocul Pharmacol Ther. 2013;29:290–7.
Realini T, Nguyen QH, Katz G, Dubiner H. Fixed-combination brinzolamide 1%/brimonidine 0.2% vs monotherapy with brinzolamide or brimonidine in patients with open-angle glaucoma or ocular hypertension: results of a pooled analysis of two phase 3 studies. Eye (Lond). 2013;27:841–7.
Aung T, Laganovska G, Hernandez Paredes TJ, Branch JD, Tsorbatzoglou A, Goldberg I. Twice-daily brinzolamide/brimonidine fixed combination versus brinzolamide or brimonidine in open-angle glaucoma or ocular hypertension. Ophthalmology. 2014. [Epub ahead of print].
Webers CA, Beckers HJ, Nuijts RM, Schouten JS. Pharmacological management of primary open-angle glaucoma: second-line options and beyond. Drugs Aging. 2008;25:729–59.
Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29:312–34.
Gellad WF, Haas JS, Safran DG. Race/ethnicity and nonadherence to prescription medications among seniors: results of a national study. J Gen Intern Med. 2007;22:1572–8.
Sleath B, Robin AL, Covert D, Byrd JE, Tudor G, Svarstad B. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology. 2006;113:431–6.
Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29:618–21.
Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153(1–9):e2.
Olthoff CM, Schouten JS, van de Borne BW, Webers CA. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005;112:953–61.
Houde M, Castilloux AM, Tingey D, Assalian A, LeLorier J. Prescription of topical antiglaucoma agents for patients with contraindications to beta-blockers. Can J Ophthalmol. 2003;38:469–75.
Vinker S, Kaiserman I, Waitman DA, Blackman S, Kitai E. Prescription of ocular beta-blockers in patients with obstructive pulmonary disease: does a central electronic medical record make a difference? Clin Drug Investig. 2006;26:495–500.
Acknowledgments
All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. This study and the associated article processing charges were funded by Alcon Laboratories, Inc. (Fort Worth, TX, USA) and presented in part at the European Glaucoma Society Annual Congress (June 7–11, 2014; Nice, France). Medical writing assistance was provided by Jillian Gee, PhD, CMPP, and Amanda Kelly, MPhil, MSHN, of Complete Healthcare Communications, Inc. (Chadds Ford, PA, USA), and was funded by Alcon. Douglas A. Hubatsch, MSc, of Alcon Laboratories, Inc., provided critical review of the manuscript.
Conflict of interest
Stefano A. Gandolfi has received grants from Alcon Laboratories, Inc.; Allergan; Glaukos Corporation; Ivantis, Inc.; Novartis; the Italian Ministry of Education; and Regione Emilia Romagna and has received personal fees from Alcon Laboratories, Inc.; Allergan; Sensimed AG; Novartis; and Merck Sharp & Dohme. Juan Camilo Parra Restrepo has received personal fees from Allergan. John Lim, Ana Cristina Sanseau, and Thomas Hamacher declare that they have no conflicts of interest.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: ClinicalTrials.gov #NCT01309204.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gandolfi, S.A., Lim, J., Sanseau, A.C. et al. Randomized Trial of Brinzolamide/Brimonidine Versus Brinzolamide Plus Brimonidine for Open-Angle Glaucoma or Ocular Hypertension. Adv Ther 31, 1213–1227 (2014). https://doi.org/10.1007/s12325-014-0168-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-014-0168-y