Abstract
Ciclosporin is associated with significant toxicity, including nephrotoxicity, and with an increased risk of cardiovascular events. Many attempts have been made to wean patients from ciclosporin. Before the availability of new immunosuppressive drugs, the acute rejection rate observed after ciclosporin withdrawal did not permit the widespread use of withdrawal regimens even though meta-analysis did not show that they adversely affected patient or graft survival. Nevertheless, maintenance therapy with azathioprine and corticosteroids has not become routine practice. The introduction of mycophenolate mofetil and subsequently sirolimus has increased the number of clinical studies of the effects of ciclosporin withdrawal.
In stable patients, this withdrawal is associated with a small but significant increase in the incidence of acute rejection episodes. Declining renal function and other forms of ciclosporin-related toxicity have improved. However, this improvement was also observed when ciclosporin was only reduced (and not withdrawn), which did not increase the risk of acute rejection. More precise definition of the patients who could benefit from ciclosporin-withdrawal may help to optimise the immunosuppressive regimen in this setting.
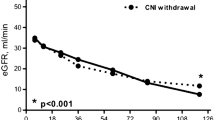
In patients with chronic allograft deterioration, ciclosporin withdrawal together with mycophenolate mofetil introduction has been shown to improve renal function significantly in many small studies, and a large prospective randomised study. For the time being, ciclosporin withdrawal is a good therapeutic option for patients with declining renal function and signs of chronic ciclosporin nephrotoxicity on renal biopsy.
Finally, recent preliminary studies have reported the results of complete avoidance of calcineurin inhibitors after renal transplantation. These results are promising as regards the incidence of acute rejection, renal function and safety, but need confirmation in larger trials with a longer follow-up.
Nevertheless, it has become clear that the concept of an immunosuppressive regimen with little or no nephrotoxicity after renal transplantation is more and more important and plays a crucial part in tailoring immunosuppression to the needs of specific patient populations.
Similar content being viewed by others
References
Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci 1993; 696: 9–19
Schreiber SL. Immunophilin-sensitive protein phosphatase action in cell signaling pathways. Cell 1992; 70(3): 365–8
Erlanger BF. Do we know the site of action of cyclosporin? Immunol Today 1992; 13(12): 487–90
Suthanthiran M, Morris RE, Strom TB. Immunosuppressants: cellular and molecular mechanisms of action. Am J Kidney Dis 1996; 28(2): 159–72
The Canadian Multicentre Transplant Study Group. A randomized clinical trial of cyclosporine in cadaveric renal transplantation: analysis at three years. N Engl J Med 1986; 314 (19): 1219–25
Myers BD, Sibley R, Newton L, et al. The long-term course of cyclosporine-associated chronic nephropathy. Kidney Int 1988; 33(2): 590–600
Goldstein DJ, Zuech N, Sehgal V, et al. Cyclosporine-associated end-stage nephropathy after cardiac transplantation: incidence and progression. Transplantation 1997; 63(5): 664–8
Bennett WM, DeMattos A, Meyer MM, et al. Chronic cyclosporine nephropathy: the Achilles’ heel of immunosuppressive therapy. Kidney Int 1996; 50(4): 1089–100
Fioretto P, Steffes MW, Mihatsch MJ, et al. Cyclosporine associated lesions in native kidneys of diabetic pancreas transplant recipients. Kidney Int 1995; 48(2): 489–95
Schorn TF, Kliem V, Bojanovski M, et al. Impact of long-term immunosuppression with cyclosporin A on serum lipids in stable renal transplant recipients. Transpl Int 1991; 4(2): 92–5
Flechner SM, Payne WD, Van Buren C, et al. The effect of cyclosporine on early graft function in human renal transplantation. Transplantation 1983; 36(3): 268–72
Remuzzi G, Perico N. Cyclosporine-induced renal dysfunction in experimental animals and humans. Kidney Int Suppl 1995; 52: S70–4
Bobadilla NA, Tapia E, Franco M, et al. Role of nitric oxide in renal hemodynamic abnormalities of cyclosporin nephrotoxicity. Kidney Int 1994; 46(3): 773–9
Bunchman TE, Brookshire CA. Cyclosporine-induced synthesis of endothelin by cultured human endothelial cells. J Clin Invest 1991; 88(1): 310–4
Lanese DM, Conger JD. Effects of endothelin receptor antagonist on cyclosporine-induced vasoconstriction in isolated rat renal arterioles. J Clin Invest 1993; 91(5): 2144–9
Palestine AG, Austin III HA, Balow JE, et al. Renal histopathologic alterations in patients treated with cyclosporine for uveitis. N Engl J Med 1986; 314(20): 1293–8
Dische FE, Neuberger J, Keating J, et al. Kidney pathology in liver allograft recipients after long-term treatment with cyclosporin A. Lab Invest 1988; 58(4): 395–402
Nizze H, Mihatsch MJ, Zollinger HU, et al. Cyclosporine-associated nephropathy in patients with heart and bone marrow transplants. Clin Nephrol 1988; 30(5): 248–60
Mihatsch MJ, Morozumi K, Strom EH, et al. Renal transplant morphology after long-term therapy with cyclosporine. Transplant Proc 1995; 27(1): 39–42
Elzinga LW, Rosen S, Bennett WM. Dissociation of glomerular filtration rate from tubulointerstitial fibrosis in experimental chronic cyclosporine nephropathy: role of sodium intake. J Am Soc Nephrol 1993; 4(2): 214–21
Shihab FS, Bennett WM, Tanner AM, et al. Angiotensin II blockade decreases TGF-beta1 and matrix proteins in cyclosporine nephropathy. Kidney Int 1997; 52(3): 660–73
Klintmalm G, Bohman SO, Sundelin B, et al. Interstitial fibrosis in renal allografts after 12 to 46 months of cyclosporin treatment: beneficial effect of low doses in early post-transplantation period. Lancet 1984; II(8409): 950–4
Burke Jr JF, Pirsch JD, Ramos EL, et al. Long-term efficacy and safety of cyclosporine in renal-transplant recipients. N Engl J Med 1994; 331(6): 358–63
Greenberg A, Thompson ME, Griffith BJ, et al. Cyclosporine nephrotoxicity in cardiac allograft patients: a seven-year follow-up. Transplantation 1990; 50(4): 589–93
O’Grady JG, Burroughs A, Hardy P, et al. Tacrolimus versus microemulsified ciclosporin in liver transplantation: the TMC randomised controlled trial. Lancet 2002; 360(9340): 1119–25
Vercauteren SB, Bosmans JL, Elseviers MM, et al. A meta-analysis and morphological review of cyclosporine-induced nephrotoxicity in auto-immune diseases. Kidney Int 1998; 54(2): 536–45
Isnard Bagnis C, Tezenas Du Montcel S, Beaufils H, et al. Long-term renal effects of low-dose cyclosporine in uveitis-treated patients: follow-up study. J Am Soc Nephrol 2002; 13(12): 2962–8
Danovitch GM. Immunosuppressant-induced metabolic toxicities. Transplant Rev 2000; 14(2): 65–81
Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant 2002; 2(9): 807–18
Mihatsch MJ, Kyo M, Morozumi K, et al. The side-effects of ciclosporine-A and tacrolimus. Clin Nephrol 1998; 49(6): 356–63
Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999; 55(2): 713–23
Yilmaz S, Tomlanovich S, Matthew T, et al. Protocol core needle biopsy and histologic chronic allograft damage index (CADI) as surrogate end point for long-term graft survival in multicenter studies. J Am Soc Nephrol 2003; 14(3): 773–9
Thervet E, Morelon E, Ducloux D, et al. A pilot study of cyclosporine withdrawal in stable renal transplant recipients after azathioprinethioprine-mycophenolate mofetil conversion. Transplant Proc 2000; 32(8): 2778
Kasiske BL, Chakkera H, Louis T, et al. Immunosuppression withdrawal in renal transplantation. Transplant Proc 2000; 32(7): 1506–7
Marsh C. Calcineurin-sparing or steroid-sparing immunosuppression in renal transplantation. Curr Opin Organ Transplant 2002; 7: 145–56
Land W, Schneeberger H, Weiss M, et al. Mycophenolate mofetil monotherapy: an optimal, safe, and efficacious immunosuppressive maintenance regimen in kidney transplant patients. Transplant Proc 2001; 33(4 Suppl.): 29S–35S
Thorp M, DeMattos A, Bennett W, et al. The effect of conversion from cyclosporine to tacrolimus on gingival hyperplasia, hirsutism and cholesterol. Transplantation 2000; 69(6): 1218–20
Pham PT, Peng A, Wilkinson AH, et al. Cyclosporine and tacrolimus-associated thrombotic microangiopathy. Am J Kidney Dis 2000; 36(4): 844–50
Bergan S, Bentdal O, Sodal G, et al. Patterns of azathioprinethioprine metabolites in neutrophils, lymphocytes, reticulocytes, and erythrocytes: relevance to toxicity and monitoring in recipients of renal allografts. Ther Drug Monit 1997; 19(5): 502–9
Opelz G. Effect of immunosuppressive therapy on graft half-life projections: The Collaborative Transplant Study. Transplant Proc 1999; 31(7A): 31S–3S
Sollinger HW. Mycophenolate mofetil. Kidney Int Suppl 1995; 52: S14–7
Halloran P, Matthew T, Tomlanovich S, et al. Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double-blind, clinical studies in prevention of rejection: The International Mycophenolate Mofetil Renal Transplant Study Groups. Transplantation 1997; 63(1): 39–47
Ojo AO, Meier-Kriesche HU, Hanson JA, et al. Mycophenolate mofetil reduces late renal allograft loss independent of acute rejection. Transplantation 2000; 69(11): 2405–9
Kahan BD. Sirolimus: a comprehensive review. Expert Opin Pharmacother 2001; 2(11): 1903–17
Kahan BD. Efficacy of sirolimus compared with azathioprinethioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet 2000; 356(9225): 194–202
MacDonald AS. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation 2001; 71(2): 271–80
Kasiske BL, Heim-Duthoy K, Ma JZ. Elective cyclosporine withdrawal after renal transplantation: a meta- analysis. JAMA 1993; 269(3): 395–400
Kasiske BL, Chakkera HA, Louis TA, et al. A meta-analysis of immunosuppression withdrawal trials in renal transplantation. J Am Soc Nephrol 2000; 11(10): 1910–7
Hall BM, Tiller DJ, Hardie I, et al. Comparison of three immunosuppressive regimens in cadaver renal transplantation: long-term cyclosporine, short-term cyclosporine followed by azathioprinethioprine and prednisolone, and azathioprinethioprine and prednisolone without cyclosporine. N Engl J Med 1988; 318(23): 1499–507
Maddux MS, Veremis SA, Bauma WD, et al. Conversion from cyclosporine to azathioprinethioprine after renal transplantation: long-term effects on renal function, rejection, and allograft survival. Transplant Proc 1988; 20(3 Suppl. 3): 152–4
Showstack J, Katz P, Amend W, et al. The association of cyclosporine with the 1-year costs of cadaver-donor kidney transplants. JAMA 1990; 264(14): 1818–23
Barclay PG, Allen RD, Stewart JH, et al. Costs of immunosuppressive therapies used in renal transplantation. Transplant Proc 1992; 24(1): 165–6
Hollander AA, van Saase JL, Kootte AM, et al. Beneficial effects of conversion from cyclosporin to azathioprinethioprine after kidney transplantation. Lancet 1995; 345(8950): 610–4
Anjum S, Andany MA, McClean JC, et al. Defining the risk of elective cyclosporine withdrawal in stable kidney transplant recipients. Am J Transplant 2002; 2(2): 179–85
Jha V, Muthukumar T, Kohli HS, et al. Impact of cyclosporine withdrawal on living related renal transplants: a single-center experience. Am J Kidney Dis 2001; 37(1): 119–24
Dubey D, Kumar A, Srivastava A, et al. Cyclosporin A withdrawal in live related renal transplantation: long-term results. Clin Transplant 2001; 15(2): 136–41
Schrama YC, Joles JA, van Tol A, et al. Conversion to mycophenolate mofetil in conjunction with stepwise withdrawal of cyclosporine in stable renal transplant recipients. Transplantation 2000; 69(3): 376–83
Smak Gregoor PJ, van Gelder T, van Besouw NM, et al. Randomized study on the conversion of treatment with cyclosporine to azathioprinethioprine or mycophenolate mofetil followed by dose reduction. Transplantation 2000; 70(1): 143–8
Smak Gregoor PJ, de Sevaux RG, Ligtenberg G, et al. Withdrawal of cyclosporine or prednisone six months after kidney transplantation in patients on triple drug therapy: a randomized, prospective, multicenter study. J Am Soc Nephrol 2002; 13(5): 1365–73
Schnuelle P, van der Heide JH, Tegzess A, et al. Open randomized trial comparing early withdrawal of either cyclosporine or mycophenolate mofetil in stable renal transplant recipients initially treated with a triple drug regimen. J Am Soc Nephrol 2002; 13(2): 536–43
Abramowicz D, Manas D, Lao M, et al. Cyclosporine withdrawal from a mycophenolate mofetil-containing immunosuppressive regimen in stable kidney transplant recipients. Transplantation 2002; 74(12): 1725–34
Radermacher J, Meiners M, Bramlage C, et al. Pronounced renal vasoconstriction and systemic hypertension in renal transplant patients treated with cyclosporin A versus FK 506. Transpl Int 1998; 11(1): 3–10
Weir MR, Klassen DK, Shen SY, et al. Acute effects of intravenous cyclosporine on blood pressure, renal hemodynamics, and urine prostaglandin production of healthy humans. Transplantation 1990; 49(1): 41–7
Nielsen FT, Ottosen P, Starklint H, et al. Kidney function and morphology after short-term combination therapy with cyclosporine A, tacrolimus and sirolimus in the rat. Nephrol Dial Transplant 2003; 18(3): 491–6
Johnson RW, Kreis H, Oberbauer R, et al. Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation 2001; 72(5): 777–86
Gonwa TA, Hricik DE, Brinker K, et al. Improved renal function in sirolimus-treated renal transplant patients after early cyclosporine elimination. Transplantation 2002; 74(11): 1560–7
Mota A, Segolini G, Legendre C, et al. Patients benefit from cyclosporine withdrawal followed by sirolimus (rapamune) maintenance therapy irrespective of baseline renal function [abstract]. Am J Transplant 2002; 2(S3): 237
Kreis H, Johnson RWG, Oberbauer R, et al. Sirolimus (rapamune) allows cyclosporine withdrawal at 3 months following transplantation resulting in a durable improvement in renal function: 2 year results of the rapamune maintenance regimen trial [abstract]. Am J Transplant 2002; 2(S3): 469
Hutchinson B, Claesson K, Mota A, et al. Quality of life in sirolimus-treated kidney transplant patients after cyclosporine elimination: 2-year results [abstract]. Am J Transplant 2002; 2(S3): 263
Stallone G, Schena A, Infante B, et al. Early withdrawal of cyclosporine (CsA) ameliorates 1-yr kidney graft function and structure in sirolimus (SRL)-treated patients [abstract]. Am J Transplant 2002; 2(S3): 393
Mourad G, Vela C, Ribstein J, et al. Long-term improvement in renal function after cyclosporine reduction in renal transplant recipients with histologically proven chronic cyclosporine nephropathy. Transplantation 1998; 65(5): 661–7
Yang CW, Ahn HJ, Kim WY, et al. Cyclosporine withdrawal and mycophenolate mofetil treatment effects on the progression of chronic cyclosporine nephrotoxicity. Kidney Int 2002; 62(1): 20–30
Shihab FS, Bennett W, Yi H, et al. Mycophenolate mofetil is beneficial in lowering the increase in transforming growth factor-b1 caused by sirolimus in a chonic nephrotoxicity model [abstract]. Am J Transplant 2002; 2(S3): 321
Weir MR, Anderson L, Fink JC, et al. A novel approach to the treatment of chronic allograft nephropathy. Transplantation 1997; 64(12): 1706–10
Ducloux D, Fournier V, Bresson-Vautrin C, et al. Mycophenolate mofetil in renal transplant recipients with cyclosporine-associated nephrotoxicity: a preliminary report. Transplantation 1998; 65(11): 1504–6
Ducloux D, Motte G, Billerey C, et al. Cyclosporin withdrawal with concomitant conversion from azathioprinethioprine to mycophenolate mofetil in renal transplant recipients with chronic allograft nephropathy: a 2-year follow-up. Transpl Int 2002; 15(8): 387–92
Weir MR, Fink JC, Hanes DS, et al. Chronic allograft nephropathy: effect of cyclosporine reduction and addition of mycophenolate mofetil on progression of renal disease. Transplant Proc 1999; 31(1-2): 1286–7
Weir MR, Ward MT, Blahut SA, et al. Long-term impact of discontinued or reduced calcineurin inhibitor in patients with chronic allograft nephropathy. Kidney Int 2001; 59(4): 1567–73
Dudley C, for the MMF Creeping Creatinine Study Group. Mycophenolate mofetil substitution for CsA is an effective and safe treatment of chronic allograft dysfunction; results of a muti-center randomized controlled study [abstract]. Am J Transplant 2002; 2Suppl. 3: 148
Dominguez J, Mahalati K, Kiberd B, et al. Conversion to rapamycin immunosuppression in renal transplant recipients: report of an initial experience. Transplantation 2000; 70(8): 1244–7
Diekmann F, Waiser J, Fritsche L, et al. Conversion to rapamycin in renal allograft recipients with biopsy-proven calcineurin inhibitor-induced nephrotoxicity. Transplant Proc 2001; 33(7-8): 3234–5
Diekmann F, Waiser J, Fritsche L, et al. Conversion to sirolimus in chronic calcineurin-inhibitor toxicity in renal transplant recipients [abstract]. Am J Transplant 2002; 2(S2): 191
Peraldi MN, Morelon E, Mamzer-Bruneel MF, et al. Renal function and pathology after switch from calcineurin-dependant drugs to sirolimus in renal transplant recipients with chronic graft nephropathy [abstract]. J Am Soc Nephrol 2001; 11: 702A
Morelon E, Stern M, Kreis H. Interstitial pneumonitis associated with sirolimus therapy in renal-transplant recipients. N Engl J Med 2000; 343(3): 225–6
Morelon E, Stern M, Israel-Biet D, et al. Characteristics of sirolimus-associated interstitial pneumonitis in renal transplant patients. Transplantation 2001; 72(5): 787–90
Hariharan S, Johnson CP, Bresnahan BA, et al. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 2000; 342(9): 605–12
Dantal J, Hourmant M, Cantarovich D, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet 1998; 351(9103): 623–8
Pascual M, Curtis J, Delmonico FL, et al. A prospective, randomized clinical trial of cyclosporine reduction in stable patients greater than 12 months after renal transplantation. Transplantation 2003; 75: 1501–5
Kreis H, Miloradovich T, Mourad G, et al. Daclizumab and mycophenolate mofetil in renal transplant recipients: two-year outcome after early reduction of cyclosporine [abstract]. Am J Transplant 2003; 3(55): 476
Zaltzman JS, Pei Y, Maurer J, et al. Cyclosporine nephrotoxicity in lung transplant recipients. Transplantation 1992; 54(5): 875–8
Wells AD, Li XC, Li Y, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med 1999; 5(11): 1303–7
Lee JI, Ganster RW, Geller DA, et al. Cyclosporine A inhibits the expression of costimulatory molecules on in vitro-generated dendritic cells: association with reduced nuclear translocation of nuclear factor kappa B. Transplantation 1999; 68(9): 1255–63
Li Y, Li XC, Zheng XX, et al. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med 1999; 5(11): 1298–302
Li Y, Zheng XX, Li XC, et al. Combined costimulation blockade plus rapamycin but not cyclosporine produces permanent engraftment. Transplantation 1998; 66(10): 1387–8
Vincenti F, Ramos E, Brattstrom C, et al. Multicenter trial exploring calcineurin inhibitors avoidance in renal transplantation. Transplantation 2001; 71(9): 1282–7
Groth CG, Backman L, Morales JM, et al. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine: Sirolimus European Renal Transplant Study Group. Transplantation 1999; 67(7): 1036–42
Kreis H, Cisterne JM, Land W, et al. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation 2000; 69(7): 1252–60
Flechner SM, Goldfarb D, Modlin C, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporine. Transplantation 2002; 74(8): 1070–6
Swanson SJ, Hale DA, Mannon RB, et al. Kidney transplantation with rabbit antithymocyte globulin induction and sirolimus monotherapy. Lancet 2002; 360(9346): 1662–4
Acknowledgements
The authors have provided no information on sources of funding or on conflicts of interest directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thervet, E., Martinez, F. & Legendre, C. Benefit-Risk Assessment of Ciclosporin Withdrawal in Renal Transplant Recipients. Drug-Safety 27, 457–476 (2004). https://doi.org/10.2165/00002018-200427070-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200427070-00003