Abstract
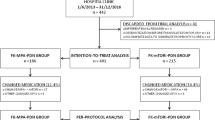
The efficacy of de novo everolimus with reduced-exposure calcineurin inhibitor (CNI) was examined in kidney transplant subpopulations from the A2309 study that were identified to be at increased risk for efficacy events. A2309 was a 24-month, multicenter, open-label trial in which 833 de novo kidney transplant recipients were randomized to everolimus targeting 3–8 or 6–12 ng/ml with reduced-exposure cyclosporine (CsA), or mycophenolic acid (MPA) with standard-exposure CsA, all with basiliximab induction. The composite efficacy endpoint was treated biopsy-proven acute rejection (BPAR), graft loss, death, or loss to follow-up. Cox proportional hazard modeling showed male gender, younger recipient age, black race, delayed graft function, human leukocyte antigen (HLA) mismatch ≥3 and increasing donor age to be significantly predictive for the composite efficacy endpoint at months 12 or 24 post-transplant. CsA exposure was 53–75 % lower, and 46–75 % lower, in patients receiving everolimus 3–8 ng/ml or receiving everolimus 6–12 ng/ml, respectively, versus MPA-treated patients. The incidence of the composite endpoint was similar in all three treatment groups within each subpopulation analyzed. The incidence of treated BPAR was similar with everolimus 3–8 ng/ml or MPA in all subpopulations, but less frequent with everolimus 6–12 ng/ml versus MPA in patients with HLA mismatch ≥3 (p = 0.049). This post hoc analysis of a large, randomized trial suggests that a de novo regimen of everolimus with reduced-exposure CsA maintains immunosuppressive efficacy even in kidney transplant patients at increased risk for efficacy events despite substantial reductions in CsA exposure.

Similar content being viewed by others
References
Flechner SM, Kobashigawa J, Klintmalm G (2008) Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant 22:1–15
Sharif A, Shabir S, Chand S, Cockwell P, Ball S, Borrows R (2011) Meta-analysis of calcineurin-inhibitor-sparing regimens in kidney transplantation. J Am Soc Nephrol 22:2107–2118
Ruiz R, Klintmalm GB (2012) Renal-sparing regimens employing new agents. Curr Opin Organ Transplant 17:619–625
Su L, Tam N, Deng R, Chen P, Li H, Wu L (2014) Everolimus-based calcineurin-inhibitor sparing regimens for kidney transplant recipients: a systematic review and meta-analysis. Int Urol Nephrol 46:2035–2044
Gurk-Turner C, Manitpisitkul W, Cooper M (2012) A comprehensive review of everolimus clinical reports: a new mammalian target of rapamycin inhibitor. Transplantation 94:659–668
Zaza G, Granata S, Tomei P, Masola V, Gambaro G, Lupo A (2014) mTOR inhibitors and renal allograft: Yin and Yang. J Nephrol 27:495–506
Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, Kramer S, Fischer W, Gschaidmeier H, Pietruck F, ZEUS Study Investigators (2011) Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial. Lancet 377:837–847
Mjörnstedt L, Sørensen SS, von Mühlen Zur B, Jespersen B, Hansen JM, Bistrup C, Andersson H, Gustafsson B, Undset LH, Fagertun H, Solbu D, Holdaas H (2012) Improved renal function after early conversion from a calcineurin inhibitor to everolimus: a randomized trial in kidney transplantation. Am J Transplant 12:2744–2753
Budde K, Sommerer C, Rath T, Reinke P, Haller H, Witzke O, Suwelack B, Baeumer D, Sieder C, Porstner M, Arns W (2014) Renal function to 5 years after late conversion of kidney transplant patients to everolimus: a randomized trial. J Nephrol [Epub ahead of print]
Tedesco Silva H Jr, Cibrik D, Johnston T, Lackova E, Mange K, Panis C, Walker R, Wang Z, Zibari G, Kim YS (2010) Everolimus plus reduced-exposure CsA versus mycophenolic acid plus standard-exposure CsA in renal-transplant recipients. Am J Transplant 10:1401–1413
Vitko S, Tedesco H, Eris J, Pascual J, Whelchel J, Magee JC, Campbell S, Civati G, Bourbigot B, Alves Filho G, Leone J, Garcia VD, Rigotti P, Esmeraldo R, Cambi V, Haas T, Jappe A, Bernhardt P, Geissler J, Cretin N (2004) Everolimus with optimized cyclosporine dosing in renal transplant recipients: 6-month safety and efficacy results of two randomized studies. Am J Transplant 4:626–635
Chan L, Greenstein S, Hardy MA, Hartmann E, Bunnapradist S, Cibrik D, Shaw LM, Munir L, Ulbricht B, Cooper M, CRADUS09 Study Group (2008) Multicenter, randomized study of the use of everolimus with tacrolimus after renal transplantation demonstrates its effectiveness. Transplantation 85:821–826
Albano L, Berthoux F, Moal MC, Rostaing L, Legendre C, Genin R, Toupance O, Moulin B, Merville P, Rerolle JP, Bayle F, Westeel PF, Glotz D, Kossari N, Lefrançois N, Charpentier B, Blanc AS, Di Giambattista F, Dantal J, RAD A2420 Study Group (2009) Incidence of delayed graft function and wound healing complications after deceased-donor kidney transplantation is not affected by de novo everolimus. Transplantation 88:69–76
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130:461–470
Cibrik D, Silva HT Jr, Vathsala A, Lackova E, Cornu-Artis C, Walker RG, Wang Z, Zibari GB, Shihab F, Kim YS (2013) Randomized trial of everolimus-facilitated calcineurin inhibitor minimization over 24 months in renal transplantation. Transplantation 95:933–942
Melancon K, Mulgaonkar SP, Delcoro C, Wiland A, McCague K, Shihab FS (2013) Outcomes in ethnic minority renal transplant recipients receiving everolimus versus mycophenolate: comparative risk assessment results from a pooled analysis. Transplantation 96:1073–1081
Boom H, Mallat MJ, de Fijter JW, Zwinderman AH, Paul LC (2000) Delayed graft function influences renal function, but not survival. Kidney Int 58:859–866
Quiroga I, McShane P, Koo DD, Gray D, Friend PJ, Fuggle S, Darby C (2006) Major effects of delayed graft function and cold ischaemia time on renal allograft survival. Nephrol Dial Transpl 21:1689–1696
Jayaram D, Kommareddi M, Sung RS, Luan FL (2012) Delayed graft function requiring more than one-time dialysis treatment is associated with inferior clinical outcomes. Clin Transplant 26:E536–E543
Yarlagadda SG, Coca SG, Formica RN Jr, Poggio ED, Parikh CR (2009) Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant 24:1039–1047
Gore JL, Pham PT, Danovitch GM, Wilkinson AH, Rosenthal JT, Lipshutz GS, Singer JS (2006) Obesity and outcome following renal transplantation. Am J Transplant 6:357–363
Cole EH, Johnston O, Rose CL, Gill JS (2008) Impact of acute rejection and new-onset diabetes on long-term transplant graft and patient survival. Clin J Am Soc Nephrol 3:814–821
Dunn TB, Noreen H, Gillingham K, Maurer D, Ozturk OG, Pruett TL, Bray RA, Gebel HM, Matas AJ (2011) Revisiting traditional risk factors for rejection and graft loss after kidney transplantation. Am J Transplant 11:2132–2143
Fan PY, Ashby VB, Fuller DS, Boulware LE, Kao A, Norman SP, Randall HB, Young C, Kalbfleisch JD, Leichtman AB (2010) Access and outcomes among minority transplant patients, 1999–2008, with a focus on determinants of kidney graft survival. Am J Transplant 10:1090–1107
Ng FL, Holt DW, Chang RWS, Macphee IA (2010) Black renal transplant recipients have poorer long-term graft survival than CYP3A5 expressers from other ethnic groups. Nephrol Dial Transplant 25:628–634
Tullius SG, Tran H, Guleria I, Malek SK, Tilney NL, Milford E (2010) The combination of donor and recipient age is critical in determining host immunoresponsiveness and renal transplant outcome. Ann Surg 252:662–674
De Fijter J, Mallat MJ, Doxiadis II, Ringers J, Rosendaal FR, Claas FH, Paul LC (2001) Increased immunogenicity and cause of graft loss of old donor kidneys. J Am Soc Nephrol 12:1538–1546
Süsal C, Döhler B, Sadeghi M, Ovens J, Opelz G (2009) HLA antibodies and the occurrence of early adverse events in the modern era of transplantation: a Collaborative Transplant Study report. Transplantation 87:1367–1371
Lim WH, Chadban SJ, Clayton P, Budgeon CA, Murray K, Campbell SB, Cohney S, Russ GR, McDonald SP (2012) Human leukocyte antigen mismatches associated with increased risk of rejection, graft failure, and death independent of initial immunosuppression in renal transplant recipients. Clin Transplant 26:E428–E437
Øien CM, Reisaeter AV, Leivestad T, Dekker FW, Line PD, Os I (2007) Living donor kidney transplantation: the effects of donor age and gender on short- and long-term outcomes. Transplantation 83:600–606
Acknowledgments
The study was funded by Novartis Pharma AG, Basel, Switzerland.
Conflicts of interest
Mario Carmellini has no conflicts of interest to declare. Valter Garcia has received travel honoraria and research grants from Novartis, Roche, Libbs and Pfizer, and research grants from Bristol Meyers Squibb and Astellas. Zailong Wang and Marcela Vergara are employees of Novartis Pharma AG. Graeme Russ has served on advisory boards for Novartis and speaker lists for Astellas and Novartis.
Research involving human participiants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Study investigators
Argentina: P Novoa, R Schiavelli, L Toselli; Australia: S Campbell, S Chadban, B Hutchinson, J Kanellis, P O’Connell, B Pussell, G Russ, R Walker; Brazil: D Carvalho, V Garcia, L Soares, H Tedesco Silva; Canada: M Walsh; Hong Kong: CS Li; Italy: A Albertazzi, M Carmellini, G Civati, G Colussi, F Schena; Korea: H Duck Jong, SJ Kim, YH Kim, YL Kim, YS Kim, IS Moon; New Zealand: H Pilmore; Slovakia: E Lackova, R Roland; Singapore: T Kee; South Africa: S Naicker; Sweden: G Tufveson; Taiwan: PH Lee; Turkey: E Akin, K Keven, A Uslu; United Kingdom: M Yacoob, H Riad, J Pattison; United States of America: S Abul-Ezz, E Alfrey, K Andreoni, M Aaronson, P Baliga, Y Becker, P Bolin, L Chan, DM Cibrik, M Cooper, A Cotterell, C Foster, C Franklin, S Jensik, TD Johnston, B Kahan, D Katz, D Kim, M Kumar, PC Kuo, J Leone, M Levy, B Marder, B Mistry, S Mulgaonkar, LL Mulloy, T O’Connor, PT Pham, K Rice, V Scantlebury, B Sankari, R Santella, H Shidban, F Shihab, D Slakey, RB Stevens, JR Thistlewaite, JD Welchel, CT Van Buren, N Youssef, C Zayas, G Zibari.
Rights and permissions
About this article
Cite this article
Carmellini, M., Garcia, V., Wang, Z. et al. Efficacy of everolimus with reduced-exposure cyclosporine in de novo kidney transplant patients at increased risk for efficacy events: analysis of a randomized trial. J Nephrol 28, 633–639 (2015). https://doi.org/10.1007/s40620-015-0180-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-015-0180-6