Abstract
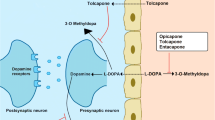
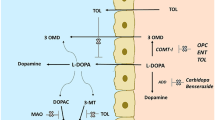
Levodopa is the cornerstone of idiopathic Parkinson’s disease (PD) treatment. However, after long-term use of levodopa, a significant percentage of patients experience motor fluctuations, which worsen their quality of life. Catechol-O-methyltransferase (COMT) inhibitors reduce levodopa metabolism and enhance the respective plasma levels, resulting in improvements in symptoms and overall quality of life.
Tolcapone was the first drug of this class to be marketed, but was withdrawn in the European Union due to its implication in the deaths of three PD patients due to hepatic failure. Three deaths from fulminant hepatic failure in 40 000 patient-years is a number that is 10–100 times higher than the expected incidence in the general population and, according to the manufacturer’s own information, the number is probably underestimated due to under-reporting of cases.
In the US, tolcapone was not withdrawn, but restrictive liver enzyme monitoring measures were issued by authorities, which severely limited its use. No further deaths from hepatic failure were reported since these measures were implemented.
The mechanisms by which tolcapone may induce liver toxicity are still under debate. It was thought that mitochondrial uncoupling of oxidative phosphorylation by tolcapone, and consequent impairment of energy production by hepatocytes, could be responsible for the observed effects.
Some experts consider that the restrictive guidelines issued in the US regarding tolcapone use may be loosened with no consequential reductions in safety. It was suggested that ongoing clinical information about safety should be considered and periodical revisions of the restrictions made accordingly. The identification of the molecular and biochemical basis of tolcapone hepatotoxicity, when completed, should also provide important indications for the clinical use of this drug.
In conclusion, appropriate monitoring of liver function can ensure adequate safety in PD patients receiving tolcapone, who can therefore benefit from the symptomatic improvements obtained with this drug.
Similar content being viewed by others
References
Ruottinen HM, Rinne UK. COMT inhibition in the treatment of Parkinson’s disease. J Neurol 1998; 245(11 Suppl. 3): 25–34
Borges N, Vieira-Coelho MA, Parada A, et al. Studies on the tight-binding nature of tolcapone inhibition of soluble and membrane-bound rat brain catechol-O-methyltransferase. J Pharmacol Exp Ther 1997; 282(2): 812–7
Zurcher G, Keller HH, Kettler R, et al. Ro 40-7592, a novel, very potent, and orally active inhibitor of catechol-O-methyltransferase: a pharmacological study in rats. Adv Neurol 1990; 53: 497–503
Zurcher G, Colzi A, Da Prada M. Ro 40-7592: inhibition of COMT in rat brain and extracerebral tissues. J Neural Transm Suppl 1990; 32: 375–80
Mannisto PT, Ulmanen I, Lundstrom K, et al. Characteristics of catechol O-methyl-transferase (COMT) and properties of selective COMT inhibitors. Prog Drug Res 1992; 39: 291–350
Nutt JG, Fellman JH. Pharmacokinetics of levodopa. Clin Neuropharmacol 1984; 7(1): 35–49
Gomes P, Soares-da-Silva P. Interaction between L-DOPA and 3-O-methyl-L-DOPA for transport in immortalised rat capillary cerebral endothelial cells. Neuropharmacology 1999; 38(9): 1371–80
Reches A, Mielke LR, Fahn S. 3-o-methyldopa inhibits rotations induced by levodopa in rats after unilateral destruction of the nigrostriatal pathway. Neurology 1982; 32(8): 887–8
Dupont E, Burgunder JM, Findley LJ, et al. Tolcapone added to levodopa in stable parkinsonian patients: a double-blind placebo-controlled study. Tolcapone in Parkinson’s Disease Study Group II (TIPS II). Mov Disord 1997; 12(6): 928–34
Roberts JW, Cora-Locatelli G, Bravi D, et al. Catechol-O-methyltransferase inhibitor tolcapone prolongs levodopa/carbidopa action in parkinsonian patients. Neurology 1993; 43(12): 2685–8
Spencer CM, Benfield P. Tolcapone. CNS Drugs 1996; 5(6): 475–81
Waters CH, Kurth M, Bailey P, et al. Tolcapone in stable Parkinson’s disease: efficacy and safety of long-term treatment. The Tolcapone Stable Study Group. Neurology 1997; 49(3): 665–71
Watkins P. COMT inhibitors and liver toxicity. Neurology 2000; 55(11 Suppl. 4): S51–2
Kurth MC, Adler CH, Hilaire MS, et al. Tolcapone improves motor function and reduces levodopa requirement in patients with Parkinson’s disease experiencing motor fluctuations: a multicenter, double-blind, randomized, placebo-controlled trial. Tolcapone Fluctuator Study Group I. Neurology 1997; 48(1): 81–7
Assal F, Spahr L, Hadengue A, et al. Tolcapone and fulminant hepatitis [letter]. Lancet 1998; 352(9132): 958
Colosimo C. The rise and fall of tolcapone. J Neurol 1999; 246(10): 880–2
European Medicine Evaluation Agency. Recommendation for the suspension of the marketing authorization for Tasmar (tolcapone) [press release]. London: EMEA, 1998
Ellison RH. Dear Healthcare Professional letter regarding appropriate use of Tasmar. Nutley (NJ): Roche Laboratories Inc, 1998 Nov 16
Roche Laboratories. Tasmar® (tolcapone tablets) prescribing information. Nutley (NJ): 1998
Spahr L, Rubbia-Brandt L, Burkhard PR, et al. Tolcapone-related fulminant hepatitis: electron microscopy shows mitochondrial alterations. Dig Dis Sci 2000; 45(9): 1881–4
Nissinen E, Kaheinen P, Penttila KE, et al. Entacapone, a novel catechol-O-methyltransferase inhibitor for Parkinson’s disease, does not impair mitochondrial energy production. Eur J Pharmacol 1997; 340(2-3): 287–94
Haasio K, Sopanen L, Vaalavirta L, et al. Comparative toxicological study on the hepatic safety of entacapone and tolcapone in the rat. J Neural Transm 2001; 108(1): 79–91
Lautala P, Ethell BT, Taskinen J, et al. The specificity of glucuronidation of entacapone and tolcapone by recombinant human UDP-glucuronosyltransferases. Drug Metab Dispos 2000; 28(11): 1385–9
New warnings for Parkinson’s drug, Tasmar. T98-81. 16-11-1998. Rockville (MD): Food and Drug Administration. Talk Paper
Olanow CW. Tolcapone and hepatotoxic effects. Tasmar Advisory Panel. Arch Neurol 2000; 57(2): 263–7
Kaakkola S. Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson’s disease. Drugs 2000; 59(6): 1233–50
Factor SA, Molho ES, Feustel PJ, et al. Long-term comparative experience with tolcapone and entacapone in advanced Parkinson’s disease. Clin Neuropharmacol 2001; 24(5): 295–9
Onofrj M, Thomas A, Iacono D, et al. Switch-over from tolcapone to entacapone in severe Parkinson’s disease patients. Eur Neurol 2001; 46(1): 11–6
Shepherd J, Clegg A. Entacapone as an adjunctive treatment to levodopa in Parkinson’s disease. Southampton, Wessex Institute for Health Research and Development. 1999. Development and Evaluation Committee Report No. 104
Rinne UK, Larsen JP, Siden A, et al. Entacapone enhances the response to levodopa in parkinsonian patients with motor fluctuations. Nomecomt Study Group. Neurology 1998; 51(5): 1309–14
Entacapone improves motor fluctuations in levodopa-treated Parkinson’s disease patients. Parkinson Study Group. Ann Neurol 1997; 42 (5): 747–55
Acknowledgements
The authors received no funding to assist in the preparation of this manuscript. The authors have provided no information on conflicts of interest directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borges, N. Tolcapone-Related Liver Dysfunction. Drug-Safety 26, 743–747 (2003). https://doi.org/10.2165/00002018-200326110-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200326110-00001