Abstract
Erythropoietin (EPO), a type I cytokine originally identified for its critical role in hematopoiesis, has been shown to have non-hematopoietic, tissue-protective effects, including suppression of atherosclerosis. However, prothrombotic effects of EPO hinder its potential clinical use in nonanemic patients. In the present study, we investigated the antiatherosclerotic effects of helix B surface peptide (HBSP), a nonerythropoietic, tissue-protective compound derived from EPO, by using human umbilical vein endothelial cells (HUVECs) and human monocytic THP-1 cells in vitro and Watanabe heritable hyperlipidemic spontaneous myocardial infarction (WHHLMI) rabbits in vivo. In HUVECs, HBSP inhibited apoptosis (≈70%) induced by C-reactive protein (CRP), a direct mediator of atherosclerosis. By using a small interfering RNA approach, Akt was shown to be a key molecule in HBSP-mediated prevention of apoptosis. HBSP also attenuated CRP-induced production of tumor necrosis factor (TNF)-α and matrix metalloproteinase-9 in THP-1 cells. In the WHHLMI rabbit, HBSP significantly suppressed progression of coronary atherosclerotic lesions as assessed by mean cross-sectional stenosis (HBSP 21.3 ± 2.2% versus control peptide 38.0 ± 2.7%) and inhibited coronary artery endothelial cell apoptosis with increased activation of Akt. Furthermore, TNF-α expression and the number of M1 macrophages and M1/M2 macrophage ratio in coronary atherosclerotic lesions were markedly reduced in HBSP-treated animals. In conclusion, these data demonstrate that HBSP suppresses coronary atherosclerosis, in part by inhibiting endothelial cell apoptosis through activation of Akt and in association with decreased TNF-α production and modified macrophage polarization in coronary atherosclerotic lesions. Because HBSP does not have the prothrombotic effects of EPO, our study may provide a novel therapeutic strategy that prevents progression of coronary artery disease.
Similar content being viewed by others
Introduction
Erythropoietin (EPO) is a critical regulator of hematopoiesis, and recombinant human (rh) EPO is widely used in the treatment of anemia associated with chronic kidney disease, heart failure and cancer (1). Over the past decade, many nonhematopoietic actions of EPO have been identified (2), including suppression of atherosclerosis (3–6). In these nonhematopoietic roles, locally produced hypoglycosylated-EPO functions in a paracrine-autocrine manner and signals through a tissue-protective receptor consisting of the EPO receptor (EPOR) and the β common receptor (βcR) (7). However, prothrombotic adverse effects of rh-EPO hinder its clinical use in nonanemic patients (8–10). To prevent thrombotic events associated with EPO therapy, the engineering of nonerythropoietic, tissue-protective EPO derivatives has been undertaken. Helix B surface peptide (HBSP) is one such EPO derivative that mimics the aqueous face of helix B of EPO and exhibits tissue-protective activities comparable with rhEPO (11-14).
Developing a novel therapeutic strategy for coronary atherosclerosis is a critical issue because coronary artery disease (CAD) remains the leading cause of mortality in developed countries. Previous work demonstrated that administration of EPO decreased cholesterol ester content of the aorta in Watanabe heritable hyperlipidemic (WHHL) rabbits (3) and intima-media thickness in hemodialyzed patients (4). Furthermore, EPO has been shown to suppress foam cell formation in murine macrophages (6). However, it is currently unknown whether nonerythropoietic derivatives of EPO such as HBSP have a protective effect against progression of coronary atherosclerotic lesions.
In the present study, we examined the antiatherosclerotic effects of HBSP by using human umbilical vein endothelial cells (HUVECs) and human monocytic THP-1 cells in vitro and Watanabe heritable hyperlipidemic spontaneous myocardial infarction (WHHLMI) rabbits in vivo. The WHHLMI rabbit is an animal model that develops spontaneous myocardial infarction mimicking that observed in humans and is useful for investigating progression of coronary atherosclerotic lesions (15). Administration of HBSP resulted in Akt-mediated inhibition of endothelial cell apoptosis, reduction of TNF-α production and modification of macrophage polarization, leading to a significant suppression of progression of coronary atherosclerotic lesions. These data confirm the protective effects of HBSP against coronary atherosclerosis as a novel therapeutic alternative to EPO for treatment of CAD.
Materials and Methods
Materials
HBSP (QEQLERALNSS) and a control, scrambled peptide (LSEQARNQSEL) were purchased from commercial manufacturers. Recombinant human EPO was obtained from Kyowa Hakko Kirin (Tokyo, Japan). Sodium azide-free recombinant human CRP was purchased from Oriental Yeast (Tokyo, Japan), and endotoxin was removed as previously described (16). Antibodies used were as follows: anti-CD31 (Dianova, Hamburg, Germany); anti-rabbit CRP (Immunology Consultants Laboratory, Newberg, OR, USA); anti-MMP-9 (Daiichi Fine Chemical, Toyama, Japan); anti-ERK2, anti-F4/80, anti-CD11c, anti-CD206, anti-IL10, anti-TNF-α and anti-EPOR (sc-82593) (Santa Cruz Biotechnology, Santa Cruz, CA, USA); and anti-Akt, anti-phospho-Akt, anti-phospho-ERK1/2, anti-FoxO3a, anti-phospho-FoxO3a, anti-eNOS, anti-phospho-eNOS and anti-β-actin (Cell Signaling Technology, Danvers, MA, USA). All other materials were from Sigma-Aldrich (St. Louis, MO, USA) except where indicated.
Animals
WHHLMI rabbits (eight males and eight females, 2 months of age, 2.2 ± 0.06 kg) were housed individually in metal cages in a room with constant temperature (22 ± 2°C) and a regular lighting cycle (12 h light/dark) and were fed standard rabbit chow (120 g/d; CR-3, Clea Japan, Tokyo, Japan) and water (ad libitum) at the Institute for Experimental Animals, Kobe University Graduate School of Medicine. All experiments were approved by the Institutional Animal Care and Use Committee of Kobe University in accordance with the directives of the Guidelines for Proper Conduct of Animal Experiments of the Science Council of Japan.
Cell Culture
HUVECs were purchased from Cell Applications (San Diego, CA, USA) and grown in medium EGM-2 supplemented with 2% fetal calf serum and penicillin/streptomycin at 37°C under air containing 5% CO2 in a humidified incubator. THP-1 cells (ATCC, Manassas, VA, USA) were maintained in medium RPMI 1640 supplemented with 10% fetal calf serum and penicillin/streptomycin. Before experiments, THP-1 cells were treated with 100 nmol/L PMA for 12 h to induce characteristics of macrophages.
Determination of Apoptosis by Flow Cytometric Analysis
Apoptosis of HUVECs was assessed by using the TACS Annexin V-FITC Apoptosis Detection Kit (R&D Systems, Minneapolis, MN, USA) as previously described (13). The percentage of annexin V-positive cells determined over 10,000 acquired events was analyzed by a FACSCalibur system equipped with a 488-nm argon laser and CellQuest software (BD Biosciences, San Jose, CA, USA).
Small Interfering RNA Transfection
Small interfering RNA (siRNA) against Akt1 (Akt siRNA) and ERK1 (ERK siRNA) were obtained from Vicgene (Mountain View, CA, USA), and scrambled siRNA was purchased from Ambion (Austin, TX, USA). HUVECs were transfected with Akt siRNA, ERK siRNA or scrambled siRNA at a concentration of 20 nmol/L by using a Silencer siRNA Transfection II Kit (Ambion). CRP 95.2 nmol/L [10 mg/L]), CRP and HBSP (2.0 nmol/L) or vehicle was added to cultures 24 h after transfection, and cells were incubated for an additional 24 h. Then cells were harvested and apoptosis was assessed by flow cytometric analysis.
Western Blot Analysis
After HUVECs were treated with HBSP, EPO or a scrambled peptide, cell lysates were prepared and Western blot was performed as previously described (17). Briefly, cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ, USA). The membrane was blocked for 1 h at room temperature with phosphate-buffered saline containing 2% bovine serum albumin and 0.05% Tween 20. The blots were incubated overnight at 4°C or for 4 h at room temperature with a primary antibody against phospho-Akt, phospho-ERK1/2, phospho-FoxO3a or phospho-eNOS followed by incubation for 1 h with a secondary, horseradish peroxidase-conjugated antibody. Then the blots were reprobed with an antibody against Akt, ERK2, FoxO3a or eNOS to show equal protein loading. In siRNA experiments, HUVECs were transfected with Akt siRNA or scrambled siRNA for 24 h. Then a Western blot was performed by using an antibody against Akt or β-actin. Immunoreactive bands were visualized by using enhanced chemiluminescence (ECL) (Amersham Biosciences). Densitometric analysis of immunoreactive bands was performed by using NIH ImageJ to assess the levels of protein expression. All experiments were performed at least three times.
Measurement of Production of TNF-α and Matrix Metalloproteinase-9 (MMP-9) in THP-1 Cells
THP-1 cells were incubated with CRP (47.6 nmol/L [5 mg/L]), CRP + EPO (10,000 IU/L), CRP + HBSP (2.0 nmol/L) or vehicle for 24 h, and then supernatants were withdrawn from the culture dishes and stored at −80°C. Concentrations of TNF-α and MMP-9 in the culture supernatants were measured as previously described (16).
In Vivo Experimental Protocol
HBSP or a control peptide was administered to WHHLMI rabbits (n = 8 each group) by subcutaneous injection at a dosage of 30 µg/kg body weight, three times a week for 32 wks. Blood was drawn every 4 wks from the marginal ear vein for blood chemical analysis. Before death, animals were given intravenous injection of sodium pentobarbital (25 mg/kg) and perfused with lactated Ringer’s solution. Then the heart and the aorta were removed, fixed with 10% buffered formalin and embedded in paraffin for histological analysis. One rabbit treated with the control peptide was excluded from the study because of poor feeding.
Histological Analysis
By using methods described previously (15,18), the degree of coronary atherosclerosis was assessed by percent cross-sectional stenosis (lesion area/area circumscribed by internal elastic lamina) by using the left circumflex artery, and the degree of aortic atherosclerosis was evaluated by percent lesion area on the surface of the whole intima (surface lesion area/whole surface intimal area). Immunohisto-chemistry and in situ apoptosis detection were performed as previously described (13). Briefly, an LSAB+ Kit (DAKO, Glostrup, Denmark) was used for the immunostaining of the deparaffinized sections with diaminobenzidine as the chromogen, and nuclei were counterstained with hematoxylin. The number of cells or area stained was calculated by using NIH ImageJ. In situ terminal deoxynucleotidyl transferase (TdT) assay was performed to detect apoptotic cells by using an ApopTag Plus In Situ Apoptosis Detection Kit (Chemicon, Billerica, MA, USA) according to the manufacturer’s instructions. Positive-control sections were from rat mammary gland. Endothelial cells were detected as CD31, also known as PECAM-1, positive cells. The number of endothelial cells positive for phospho-Akt, phospho-ERK1/2 or TdT labeling was determined in a blinded fashion by counting whole endothelial cells in each section on a digital microscopic system (BX51/DP72; Olympus, Tokyo, Japan) at 400x magnification. Immunostaining of F4/80, CD11c, CD206, IL-10, TNF-α, MMP-9, CRP and EPOR was performed by using an LSAB+ Kit, as mentioned above.
Blood Chemical Analysis
Serum levels of total cholesterol, triglyceride, TNF-α and CRP were determined by enzymatic methods and enzyme-linked immunosorbent assay (ELISA). Complete blood cell counts were measured with an automated hematological analyzer.
Statistical Analysis
Data are shown as mean ± standard error of the mean (SEM). Differences were analyzed with Student t tests or Mann-Whitney U test between two groups and one-way analysis of variance (ANOVA) or Kruskal-Wallis test among more than three groups, as appropriate. Values of P < 0.05 were considered statistically significant.
All supplementary materials are available online at https://doi.org/www.molmed.org .
Results
Apoptosis of HUVECs and the Role of Akt
Growing evidence has shown that CRP is not just a predictor of CAD but a direct mediator of atherosclerosis (16,19–21). Consistent with previous work (16), CRP (95.2 nmol/L [10 mg/L]) induced apoptosis in HUVECs (Figure 1A). Simultaneous treatment with HBSP (2.0 nmol/L, the molar equivalent of 10,000 IU/L of EPO, and 20 nmol/L) clearly decreased the number of apoptotic cells induced by CRP, to a greater degree than EPO (10,000 IU/L) (see Figure 1A). To examine the mechanisms by which HBSP inhibits HUVEC apoptosis, we investigated intracellular signaling pathways for cell survival. HBSP, as well as EPO, activated Akt and ERK1/2 in HUVECs with a peak at 15 min after incubation (Figure 1B) and a maximum response occurring for a concentration of HBSP of 2 nmol/L (Figure 1C). To clarify which pathway is critical for inhibiting apoptosis, we examined the effects of Akt siRNA and ERK siRNA on HBSP-induced inhibition of apoptosis. Akt siRNA completely abolished the inhibition of apoptosis induced by HBSP in HUVECs (Figure 1D). In contrast, scrambled siRNA and ERK siRNA did not affect the inhibitory effect of HBSP on HUVEC apoptosis (see Figure 1D). Western blot analysis demonstrated a significant downregulation of Akt by Akt siRNA at the protein level (Figure 1E). In addition, HBSP and EPO also phosphorylated FoxO3 and eNOS, downstream targets of Akt, which may confer protection against apoptosis (Supplementary Figure S1).
HBSP and EPO inhibit HUVEC apoptosis via activation of molecular pathways increasing cell survival. (A) HBSP and EPO reduce CRP-induced HUVEC apoptosis. HUVECs were treated with vehicle, CRP (95.2 nmol/L), CRP + EPO (10,000 IU/L) or CRP + HBSP (2.0 or 20 nmol/L) for 24 h. Then cells were harvested by trypsinization, and flow cytometric analysis was performed to evaluate apoptosis. Data are mean ± SEM, n = 18 each group; *P < 0.01 versus CRP#P < 0.01 versus CRP + EPO. (B) HBSP and EPO activate Akt and ERK1/2. HUVECs were incubated with a control peptide (C; a scrambled version of HBSP 2.0 nmol/L, 15 min), HBSP (2.0 nmol/L) or EPO (10,000 IU/L) for the indicated times, and cell lysates were prepared. Next, Western blot was performed as described in Materials and Methods. Immunoreactive bands were analyzed by densitometry by using NIH ImageJ, and ratios of the phospho/total antibody immunolabeling were calculated. The ratios were compared between C and 15 min in HBSP and between 0 and 15 min in EPO. The pAkt/Akt ratios at 30 min in EPO were also calculated. Data are mean ± SEM, n = 3 each group; *P < 0.01, #P < 0.05. (C) A similar analysis performed after incubation in increasing concentrations of EPO or HBSP showed that a peak phosphorylation of Akt occurred at ~2 nmol/L. (D) Akt is required for HBSP-mediated inhibition of apoptosis. Akt siRNA, ERK siRNA or scrambled siRNA was transfected into HUVECs as described in Materials and Methods. Then apoptosis of HUVECs was evaluated as in (A). A, control + scrambled siRNA; B, CRP + scrambled siRNA; C, CRP + HBSP + scrambled siRNA; D, CRP + HBSP + Akt siRNA; E, CRP + HBSP + ERK siRNA. Data are mean ± SEM, n = 8 each group; *P < 0.05 versus CRP + scrambled siRNA, #P < 0.05 versus CRP + HBSP + scrambled siRNA and CRP + HBSP + ERK siRNA. (E) Akt is downregulated by Akt siRNA at the protein level. Akt siRNA or scrambled siRNA was transfected into HUVECs in the same manner as in (D), without CRP or HBSP treatment, and cell lysates were prepared. Next, Western blot was performed as described in Materials and Methods by using an antibody against Akt or β-actin (a loading control). Immunoreactive bands were analyzed by NIH ImageJ, and ratios of the Akt/β-actin antibody immunolabeling were calculated. Data are mean ± SEM, n = 3 each group; *P < 0.05.
Production of TNF-α and MMP-9 in THP-1 Cells
CRP (47.6 nmol/L [5 mg/L]) induced production of TNF-α and MMP-9 in THP-1 cells, and simultaneous treatment with HBSP (2.0 nmol/L) significantly reduced production of TNF-α and MMP-9, which was comparable to the effect of EPO (10,000 IU/L) (Figure 2).
HBSP inhibits TNF-α and MMP-9 production in THP-1 cells. (A) HBSP reduces TNF-α expression in CRP-stimulated THP-1 cells. THP-1 cells were incubated in serum-free medium with vehicle, CRP (47.6 nmol/L), CRP + EPO (10,000 IU/L) or CRP + HBSP (2.0 nmol/L) for 24 h. Then samples were collected and concentrations of TNF-α in the culture supernatants were measured by ELISA. Data are mean ± SEM, n = 6; *P < 0.01 versus CRP#P < 0.05 versus CRF? (B) HBSP reduces MMP-9 production in CRP-stimulated THP-1 cells. Cells were incubated, as in (A), and MMP-9 concentrations were measured in the same manner as in (A). Data are mean ± SEM, n = 4; *P < 0.05 versus CRP.
Suppression of Coronary Atherosclerosis in the WHHLMI Rabbit
The results of pharmacokinetic evaluation of related peptide pHBSP (11) administered subcutaneously showed that a dose of 30 µg/kg produced a peak plasma level of pHBSP of ~2 nmol/L (i.e., equivalent to the maximum observed for Akt phosphorylation using HUVECs in vitro) (Figure 1C). Therefore, this dose was used for the in vivo experiments.
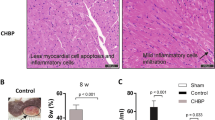
To examine the effects of HBSP on coronary atherosclerosis, 30 µg/kg body weight HBSP or a control scrambled peptide was administered subcutaneously three times a week to WHHLMI rabbits for 32 wks. All animals survived the study period. Coronary atherosclerosis, as assessed by mean cross-sectional stenosis and significant stenosis (>75%), was clearly suppressed in animals treated with HBSP compared with those treated with the control scrambled peptide (Figure 3). On the basis of the data obtained from the in vitro experiments for HUVEC apoptosis and signaling pathways of HBSP, we evaluated apoptosis and Akt signaling in coronary artery endothelial cells of WHHLMI rabbits. Administration of HBSP significantly inhibited endothelial cell apoptosis with increased Akt activation compared with the control scrambled peptide (Figure 4), whereas levels of ERK1/2 activation were not significantly different between the two groups (control 11.0 ± 1.7% versus HBSP 14.1 ± 0.9%). Next, we assessed the effect of HBSP on TNF-α expression in coronary atherosclerotic lesions. TNF-α expression was markedly reduced in animals treated with HBSP compared with those treated with the control scrambled peptide (Figure 5). In contrast, administration of HBSP had no effects on MMP-9 expression and CRP deposition in coronary atherosclerotic lesions (MMP-9 positive area, control 6.8 ± 2.4% versus HBSP 6.0 ± 3.1%; CRP positive area, control 0.037 ± 0.019% versus HBSP 0.031 ± 0.014%). Further, we evaluated the number of macrophages and the expressions of cell markers for M1 and M2 macrophages in coronary atherosclerotic lesions. The number of macrophages (F4/80-positive cells) and CD11c-positive M1 macrophages was significantly decreased in animals treated with HBSP compared with those treated with the control scrambled peptide (Figures 6A, B). Although the number of CD206-positive M2 macrophages did not differ significantly between the two groups (control 328.8 ± 77.8 cells/mm2 versus HBSP 262.9 ± 42.7 cells/mm2), M1/M2 ratio was markedly suppressed in animals treated with HBSP (Figure 6C). Also, HBSP clearly upregulated the expression of IL-10, an antiinflammatory cytokine (Figure 6D).
HBSP suppresses the formation of atherosclerotic lesions within coronary artery segments of WHHLMI rabbits. (A) HBSP reduces mean cross-sectional arterial stenosis. Left circumflex arteries were collected from WHHLMI rabbits (control, n = 7; HBSP, n = 8) and were sectioned at 500-µm intervals. The percent cross-sectional stenosis was calculated as described in Materials and Methods. The mean number of arterial segments obtained was 20.7 ± 1.4 in control animals and 16.5 ± 0.9 in animals treated with HBSP. Data are mean ± SEM; *P < 0.01. (B) HBSP reduces atheromata (that is, those causing >75% significant stenosis of the vessel lumen). On the basis of the data obtained from (A), percentage of intima with significant atheromata was calculated. Data are mean ± SEM; *P< 0.01.
HBSP inhibits apoptosis and activates Akt signaling in coronary artery endothelial cells of WHHLMI rabbits. (A) HBSP reduces apoptosis of coronary artery endothelial cells. Immunostaining of the deparaffinized sections were performed by using anti-CD31 antibody to detect endothelial cells, and an in situ TdT assay was performed. Then the percentage of endothelial cells positive for TdT labeling was determined, as described in Materials and Methods. Data are mean ± SEM; control, n = 7; HBSP n = 8; *P < 0.01. (B) HBSP activates Akt in coronary artery endothelial cells. The deparaffinized sections were incubated with a primary antibody against phospho-Akt or negative control IgG and immunostaining was performed. Then the percentage of endothelial cells positive for phospho-Akt was determined as in (A). Data are mean ± SEM; negative control IgG, n = 3; control, n = 7; HBSP n = 8; *P < 0.01.
HBSP reduces TNF-α expression in coronary atherosclerotic lesions of WHHLMI rabbits. (A) Section from control animal immunostained with negative control IgG. (B) Section from control animal immunostained with anti-TNF-α antibody. (C) Section from HBSP-treated animal immunostained with anti-TNF-α antibody. TNF-α-positive areas are visualized in brown (arrowheads). (D) Ratios of TNF-α-positive area/intimal area. Mean ± SEM; negative control IgG, n = 4; control, n = 7; HBSP, n = 8; *P < 0.01. Scale bar, 200 µm.
HBSP reduces the number and polarization of macrophages and increases IL-10 expression in coronary atherosclerotic lesions of WHHLMI rabbits. HBSP reduces the number of macrophages (A: F4/80-positive cells), M1 macrophages (B: CD11c-positive cells) and M1/M2 macrophage ratio (C) and increases IL-10-positive cells (D). Data are mean ± SEM; negative control IgG, n = 3; control, n = 7; HBSP, n = 8; *P< 0.01, #P< 0.05.
With respect to the degree of aortic atherosclerosis as assessed by percent lesion area on the surface of whole intima, there was no significant difference between the two groups (control 79.6 ± 3.5% versus HBSP 81.4 ± 2.4%). To clarify why HBSP did not inhibit aortic atherosclerosis despite clear suppression of coronary atherosclerosis, we investigated expression of EPOR in aortas and coronary arteries. As shown in Figure 7, EPOR expression was much more prominent in coronary arteries compared with aortas.
EPOR is expressed in atherosclerotic lesions of coronary arteries and aortas in WHHLMI rabbits. (A) Coronary artery section from HBSP-treated animal immunostained with negative control IgG. (B) Aorta section from HBSP-treated animal immunostained with anti-EPOR antibody. (C) Coronary artery section from HBSP-treated animal immunostained with anti-EPOR antibody. EPOR-positive areas are visualized in brown (arrowheads). (D) Ratios of EPOR-positive area/intimal area. Data are mean ± SEM; negative control IgG, n = 4; aorta, n = 8; coronary artery, n = 8; *P < 0.01. Scale bar, 200 µm.
With regard to blood chemical analysis at 32 wks after administration of HBSP or the control scrambled peptide, there were no significant differences in serum levels of total cholesterol (control 26.4 ± 2.4 mmol/L versus HBSP 25.6 ± 1.7 mmol/L), triglyceride (control 3.6 ± 0.4 mmol/L versus HBSP 3.9 ± 0.5 mmol/L), TNF-α (control 29.3 ± 1.1 pmol/L versus HBSP 30.2 ± 0.9 pmol/L), CRP (control 138.1 ± 47.6 nmol/L versus HBSP 164.8 ± 36.2 nmol/L) and hemoglobin levels (control 145 ± 4.0 g/L versus HBSP 141 ± 4.0 g/L) between the two groups.
Discussion
Recent work has shown that EPO and its nonerythropoietic derivatives, including HBSP, provide tissue-protective effects in a wide variety of tissues, in part by inhibiting apoptosis and by counteracting proinflammatory cytokines such as TNF-α (13,22–26). Consistent with these observations, our data obtained from the in vitro experiments clearly demonstrate that HBSP, as well as EPO, reduces HUVEC apoptosis and THP-1 production of TNF-α and MMP-9 induced by CRP, a direct mediator of atherosclerosis (16,19–21). Because endothelial cell apoptosis and increased production of TNF-α and MMP-9 contribute to the development and progression of atherosclerotic lesions (27–29), these results confirm the antiatherosclerotic effects of HBSP and EPO. With respect to the signaling pathways of HBSP and EPO in HUVECs, HBSP, as well as EPO, activates Akt and ERK1/2, and Akt has been shown to play an important role in HBSP-mediated inhibition of apoptosis, as assessed by an siRNA approach. Recently, we have demonstrated that Akt is a key molecule for inhibition of apoptosis mediated by HBSP in cultured rat cardiomyocytes (13). Given these observations and previous findings of EPO signaling pathways (30–32), it is likely that Akt plays a critical role in prevention of apoptosis mediated by EPO and its nonerythropoietic derivatives.
Based on the results obtained from the in vitro experiments, we examined the in vivo antiatherosclerotic effects of HBSP against progression of coronary atherosclerotic lesions by using the WHHLMI rabbit (15). This rabbit strain is characterized by the high incidence (≈97%) of spontaneous myocardial infarction at ages 11–35 months, with severe atheromatous plaques in the coronary arteries. The data from mean cross-sectional stenosis and significant stenosis (>75%) clearly show a significant suppression of progression of coronary atherosclerotic lesions that is not associated with changes in serum lipid profiles in animals treated with HBSP. These antiatherosclerotic effects of HBSP are comparable with previous findings observed for statins (18,33). Furthermore, administration of HBSP is associated with increased activation of Akt, which significantly inhibits endothelial cell apoptosis in coronary atherosclerotic lesions, suggesting a critical role of Akt in preventing apoptosis in vivo. Also, TNF-α expression within coronary atherosclerotic lesions is markedly reduced in HBSP-treated animals. These results are consistent with previous studies showing pleiotropic, tissue-protective effects of EPO and its nonerythropoietic derivatives (12–14,24–26). In addition, HBSP significantly decreases the number of M1 macrophages and the M1/M2 ratio, with increased expression of IL-10 in coronary atherosclerotic lesions. Because M1 macrophages are proinflammatory and an increased M1/M2 ratio in the vascular wall is associated with atherosclerosis (34), modification of macrophage polarization may be one of the mechanisms for the antiatherosclerotic effects of HBSP.
It is notable that expression of EPOR in atherosclerotic lesions is different between coronary arteries and aortas in the WHHLMI rabbit. Recent work demonstrated that expression of EPO and EPOR was upregulated in atherosclerotic lesions of apoE−/− mice (6). Because HBSP, as well as EPO, signals through the EPOR/βcR complex, the increased expression of EPOR in coronary arteries compared with aortas could explain the antiatherosclerotic effects of HBSP in coronary atherosclerotic lesions, but not in aortic atherosclerotic lesions. Expression of βcR was not evaluated because of the lack of a suitable anti-rabbit βcR antibody. Previous work showed the similar effects of statins that predominantly suppress atherosclerotic lesions of coronary arteries compared with those of aortas (18,33). Further studies are warranted to assess the effects of chronic HBSP administration on preventing CAD.
Conclusion
We have shown antiatherosclerotic effects of HBSP by using HUVEC and THP-1 cultures in vitro and the WHHLMI rabbit, an animal model for spontaneous myocardial infarction, in vivo. Chronic administration of HBSP suppresses coronary atherosclerosis, in part by inhibiting endothelial cell apoptosis through activation of Akt and in association with decreased TNF-α production and modified macrophage polarization in coronary atherosclerotic lesions. These antiatherosclerotic effects of HBSP depend on the regional expression of EPOR in atherosclerotic lesions. Because HBSP does not have the prothrombotic effects of EPO, administration of HBSP may provide a novel therapeutic strategy that suppresses progression of CAD.
Disclosure
M Brines, M Yamin and A Cerami are employees of Araim Pharmaceuticals, which is developing tissue-protective compounds. These authors and M Kawakami also own stock or stock options in this company. The authors declare that they have no other competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
Jelkmann W. (2007) Erythropoietin after a century of research: younger than ever. Eur. J. Haematol. 78:183–205.
Brines M, Cerami A. (2008) Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J. Intern. Med. 264:405–32.
Buemi M, et al. (1998) Does erythropoietin administration affect progression of atherosclerosis in Watanabe heritable hyperlipaemic rabbits? Nephrol. Dial. Trans-plant. 13:2706–8.
Pawlak K, Pawlak D, Mysliwiec M. (2006) Long-term erythropoietin therapy decreases CC-chemokine levels and intima-media thickness in hemodialyzed patients. Am. J. Nephrol. 26:497–502.
Siamopoulos KC, et al. (2006) Long-term treatment with EPO increases serum levels of high-density lipoprotein in patients with CKD. Am. J. Kidney Dis. 48:242–9.
Lu KY, et al. (2010) Erythropoietin suppresses the formation of macrophage foam cells: role of liver X receptor alpha. Circulation. 121:1828–37.
Brines M, et al. (2004) Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc. Natl. Acad. Sci. U. S. A. 101:14907–12.
Khorana AA, Francis CW, Culakova E, Lyman GH. (2005) Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 104:2822–9.
Corwin HL, et al. (2007) Efficacy and safety of epoetin alfa in critically ill patients. N. Engl. J. Med. 357:965–76.
Aapro M, Scherhag A, Burger HU. (2008) Effect of treatment with epoetin-beta on survival, tumour progression and thromboembolic events in patients with cancer: an updated meta-analysis of 12 randomised controlled studies including 2301 patients. Br. J. Cancer. 99:14–22.
Brines M, et al. (2008) Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc. Natl. Acad. Sci. U. S. A. 105:10925–30.
Erbayraktar Z, Erbayraktar S, Yilmaz O, Cerami A, Coleman T, Brines M. (2009) Nonerythropoietic tissue protective compounds are highly effective facilitators of wound healing. Mol. Med. 15:235–41.
Ueba H, et al. (2010) Cardioprotection by a non-erythropoietic, tissue-protective peptide mimicking the 3D structure of erythropoietin. Proc. Natl. Acad. Sci. U. S. A. 107:14357–62.
Ahmet I, et al. (2011) A small nonerythropoietic helix B surface peptide based upon erythropoietin structure is cardioprotective against ischemic myocardial damage. Mol. Med. 17:194–200.
Shiomi M, Ito T, Yamada S, Kawashima S, Fan J. (2003) Development of an animal model for spontaneous myocardial infarction (WHHLMI rabbit). Arterioscler. Thromb. Vasc. Biol. 23:1239–44.
Nabata A, et al. (2008) C-reactive protein induces endothelial cell apoptosis and matrix metalloproteinase-9 production in human mononuclear cells: implications for the destabilization of atherosclerotic plaque. Atherosclerosis. 196:129–35.
Ueba H, et al. (2005) Glimepiride induces nitric oxide production in human coronary artery endothelial cells via a PI3-kinase-Akt dependent pathway. Atherosclerosis. 183:35–9.
Shiomi M, Ito T, Hirouchi Y, Enomoto M. (2001) Fibromuscular cap composition is important for the stability of established atherosclerotic plaques in mature WHHL rabbits treated with statins. Atherosclerosis 157:75–84.
Khreiss T, Jozsef L, Potempa LA, Filep JG. (2004) Conformational rearrangement in C-reactive protein is required for proinflammatory actions on human endothelial cells. Circulation. 109:2016–22.
Verma S, Szmitko PE, Ridker PM. (2005) C-reac-tive protein comes of age. Nat. Clin. Pract. Cardiovasc. Med. 2:29–36.
Pepys MB, et al. (2006) Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 440:1217–21.
Calvillo L, et al. (2003) Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc. Natl. Acad. Sci. U. S. A. 100:4802–6.
Fiordaliso F, et al. (2005) A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injury. Proc. Natl. Acad. Sci. U. S. A. 102:2046–51.
Salahudeen AK, et al. (2008) Antiapoptotic properties of erythropoiesis-stimulating proteins in models of cisplatin-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 294:F1354–65.
Agnello D, et al. (2002) Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 952:128–34.
Villa P, et al. (2003) Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J. Exp. Med. 198:971–5.
Valgimigli M, et al. (2003) Endothelial dysfunction in acute and chronic coronary syndromes: evidence for a pathogenetic role of oxidative stress. Arch. Biochem. Biophys. 420:255–61.
McKellar GE, McCarey DW, Sattar N, McInnes IB. (2009) Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat. Rev. Cardiol. 6:410–7.
de Nooijer R, et al. (2006) Lesional overexpression of matrix metalloproteinase-9 promotes intraplaque hemorrhage in advanced lesions but not at earlier stages of atherogenesis. Arterioscler. Thromb. Vasc. Biol. 26:340–6.
Siren AL, et al. (2001) Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc. Natl. Acad. Sci. U. S. A. 98:4044–9.
Bittorf T, Buchse T, Sasse T, Jaster R, Brock J. (2001) Activation of the transcription factor NF-kappaB by the erythropoietin receptor: structural requirements and biological significance. Cell Signal. 13:673–81.
Parsa CJ, et al. (2003) A novel protective effect of erythropoietin in the infarcted heart. J. Clin. Invest. 112:999–1007.
Watanabe Y, et al. (1988) Preventive effect of pravastatin sodium, a potent inhibitor of 3-hy-droxy-3-methylglutaryl coenzyme A reductase, on coronary atherosclerosis and xanthoma in WHHL rabbits. Biochim. Biophys. Acta. 960:294–302.
Chinetti-Gbaguidi G, Staels B. (2011) Macrophage polarization in metabolic disorders: functions and regulation. Curr. Opin. Lipidol 22:365–72.
Acknowledgments
We thank Takashi Ito, Satoshi Yamada, Nobue Hirayama, Harue Fukaya, Kimiko Aoki, Chie Ishikawa and Kazuko Futaka for expert technical assistance.
This work was funded in part by Grant-in-Aid for Scientific Research 20590887 from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H Ueba and M Kawakami) and by a Research Grant for Health Science from the Ministry of Health, Labor and Welfare of Japan (to M Kawakami).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit (https://doi.org/creativecommons.org/licenses/by/4.0/)
About this article
Cite this article
Ueba, H., Shiomi, M., Brines, M. et al. Suppression of Coronary Atherosclerosis by Helix B Surface Peptide, a Nonerythropoietic, Tissue-Protective Compound Derived from Erythropoietin. Mol Med 19, 195–202 (2013). https://doi.org/10.2119/molmed.2013.00037
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2013.00037