Abstract
Spinal and bulbar muscular atrophy is an X-linked motor neuron disease caused by polyglutamine expansion in the androgen receptor. Patients develop slowly progressive proximal muscle weakness, muscle atrophy and fasciculations. Affected individuals often show gynecomastia, testicular atrophy and reduced fertility as a result of mild androgen insensitivity. No effective disease-modifying therapy is currently available for this disease. Our recent studies have demonstrated that insulinlike growth factor (IGF)-1 reduces the mutant androgen receptor toxicity through activation of Akt in vitro, and spinal and bulbar muscular atrophy transgenic mice that also overexpress a noncirculating muscle isoform of IGF-1 have a less severe phenotype. Here we sought to establish the efficacy of daily intraperitoneal injections of mecasermin rinfabate, recombinant human IGF-1 and IGF-1 binding protein 3, in a transgenic mouse model expressing the mutant androgen receptor with an expanded 97 glutamine tract. The study was done in a controlled, randomized, blinded fashion, and, to reflect the clinical settings, the injections were started after the onset of disease manifestations. The treatment resulted in increased Akt phosphorylation and reduced mutant androgen receptor aggregation in muscle. In comparison to vehicle-treated controls, IGF-1–treated transgenic mice showed improved motor performance, attenuated weight loss and increased survival. Our results suggest that peripheral tissue can be targeted to improve the spinal and bulbar muscular atrophy phenotype and indicate that IGF-1 warrants further investigation in clinical trials as a potential treatment for this disease.
Similar content being viewed by others
Introduction
Spinal and bulbar muscular atrophy (SBMA) is an X-linked neurodegenerative disorder caused by expansion of a CAG repeat encoding a polyglutamine tract in the first exon of the androgen receptor (AR) gene (1). While in the normal population, the number of CAG repeats varies from about 11 to 34, SBMA patients carry expansions to more than 38 (1,2). Expansion of the polyglutamine tract in the AR confers a toxic gain of function to the mutant protein and results in its aggregation and nuclear accumulation in inclusions (3). The disease is clinically characterized by adult-onset atrophy and weakness of limb and bulbar muscles due to lower motor neuron degeneration in the spinal cord and brain-stem (4,5); recent evidence also indicates primary involvement of skeletal muscle (6,7). Signs of androgen insensitivity, such as gynecomastia and reduced fertility, are also frequently observed (8,9).
SBMA belongs to the family of polyglutamine diseases, which also includes Huntington disease, dentatorubral-pallidoluysian atrophy and six types of spinocerebellar ataxia (1),2,3,6,7 and 17) (10). Among polyglutamine diseases, SBMA is unique in that the disease is sex-specific, that is, only males are fully affected. Studies in cell culture and animal models have provided evidence that androgen is necessary in the disease pathogenesis (11,12). Consistent with this, reduction of testosterone levels ameliorates disease manifestations in male SBMA mice (13). Nevertheless, clinical trials of anti-androgen therapy in SBMA patients have thus far given mixed results (14,15). There is currently no effective disease-modifying therapy for SBMA, or for any of the other polyglutamine diseases.
Recent evidence has shown that the protein context is also important in polyglutamine disease, and protein domains other than the polyglutamine tract play a role in the toxicity. In agreement with this, models with full-length polyglutamine-expanded AR protein more accurately reflect the human disease (16,17). Toxicity of polyglutamine-expanded proteins is affected by posttranslational modifications of the disease protein, including phosphorylation (18–20). We have recently shown that in vitro insulinlike growth factor (IGF)-1 activates the phosphatidylinositol 3-kinase–Akt signaling and increases AR phosphorylation at the Akt consensus site, resulting in reduced toxicity of the mutant AR (21), and that SBMA mice genetically overexpressing a noncirculating muscle-specific isoform of IGF-1 have a less severe phenotype (22). Akt, also known as protein kinase B, is a serine/threonine-specific protein kinase that plays a key role in multiple cellular processes.
In this study, we explored the efficacy of parenteral injections of mecasermin rinfabate, recombinant human IGF-1 and IGF-1 binding protein 3 (rhIGF-1/IGFBP3), in a transgenic mouse model of SBMA. To have clinically meaningful results, we designed the study in a controlled, randomized, blinded fashion, and the treatment was started only after the onset of disease manifestations.
We found that administration of rhIGF-1/IGFBP3 increased activation of Akt and reduced mutant AR aggregation in skeletal muscle. Moreover, it improved motor function and pathology and prolonged the lifespan of SBMA mice. Our study indicates that IGF-1 mimetics may be a promising therapeutic strategy for SBMA.
Materials and Methods
Animals and Drug Treatment
The study was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (23) and was approved by the National Institute of Neurological Disorders and Stroke (NINDS) Animal Care Committee. All the experiments were performed in male mice in the F1 generation derived by crossing C57Bl6 mice with BDF1 mice. The transgenic mice expressed mutant AR with a 97 glutamine repeat (AR97Q) (11). The mice were genotyped by polymerase chain reaction (PCR) with tail DNA as previously described (11), by using a REDExtract-N-Amp Tissue PCR kit (Sigma, St. Louis, MO, USA) according to the manufacturer’s instructions. Recombinant human IGF-1 in complex with human IGFBP-3 (Iplex, mecasermin rinfabate) was provided by Insmed Inc. (Monmouth Junction, NJ, USA). The IGF-1 complex was dissolved in a stock solution at a concentration of 60 mg/mL in 50 mmol/L sodium acetate and 105 mmol/L sodium chloride (pH 5.5). A vehicle solution was used as the control. The IGF-1 solution and vehicle were stored in aliquots at −80°C until use, and once thawed, the vials were kept at 4°C for a maximum of 24 h. IGF-1 (15 mg/kg/day) or vehicle was injected intraperitoneally into mice daily starting at 10 wks of age and continuing for 6 or 10 wks, as indicated in the Results section. The daily injections and all the analyses were performed by blinded investigators.
Behavioral and Survival Analysis
Body weight assessment and the hanging wire test were conducted every week for IGF-1–treated and vehicle-treated mice. For the hanging wire test, the mouse was placed on top of a wire cage lid. The lid was shaken slightly three times to cause the mouse to grip the wires and then the lid was turned upside down approximately 20 cm above the cage floor. The latency to fall was recorded for a maximum time of 60 s (24). We assessed the gait analysis using the Gait Analysis Treadmill (Columbus Instruments) and TreadScan software (CleverSys). The treadmill consisted of a motor-driven transparent treadmill belt with an angled mirror mounted below. A high-speed digital video camera was mounted to record a ventral view of the mouse on the treadmill belt reflected off the mirror; digital video images were recorded at 100 frames per second. For each 20-s session, the video was previewed to determine a minimum of six consecutive step cycles of consistent walking for video analysis. For survival analysis, mice were observed daily and sacrificed when the mice had lost more than 30% of body weight or showed inability to move or signs of dehydration.
Biochemical Analysis
The 16-wk-old mice were anesthetized with isoflurane and sacrificed. Quadriceps muscles and spinal cord were dissected and snap frozen in liquid nitrogen. By using a polytron homogenizer, tissue samples were processed in ice-cold homogenization buffer (150 mmol/L NaCl, 50 mmol/L Tris, 2 mmol/L EDTA, 1% sodium deoxycholate, 0.5% Triton X-100, 0.1% sodium dodecyl sulfate [SDS]) containing protease inhibitor and phosphatase inhibitor cocktails (Roche). Homogenates were sonicated and pre-cleared at 4,000g for 10 min at 4°C. The soluble fraction was collected and protein concentrations were determined using the Bradford reagent (Bio-Rad). Protein lysates were separated on Tris-glycine gels, transferred to polyvinylidene fluoride membranes (both Invitrogen) and probed with the following antibodies: AR H-280 (sc-13062; Santa Cruz Biotechnology, Santa Cruz, CA, USA), phospho-Akt and total Akt (9271 and 9272, respectively; Cell Signaling), choline acetyltransferase (AB144P; Chemicon) and α-tubulin as a loading control (T6199; Sigma). Western blots were visualized with peroxidase-linked secondary antibodies (R&D Systems) by chemiluminescence detection (PerkinElmer Life Sciences). Band densities were quantified by densitometric analysis by using ImageJ software and normalized to the α-tubulin values of the respective samples. Phospho-Akt values were normalized to band intensities of both total Akt and α-tubulin.
Taqman Quantitative PCR Analysis
Total RNA was extracted from frozen quadriceps muscle by using Trizol (Invitrogen) as previously described (25). RNA (1 µg) was reverse-transcribed using the cDNA Archive Kit (Applied Biosystems) following the manufacturer’s instructions. Gene expression was measured by quantitative real-time PCR by using an ABI 9900 Sequence Detector System (Applied Biosystems). Specific assays for myogenin (Mm_00446194-m1), acetylcholine receptor ± (Chrna1) (Mm_00431629-m1), myogenic differentiation 1 (myoD) (Mm_00440387_m1) and phosphoglycerate kinase 1 (PGK1) (Mm_00435617-m1) were from Applied Biosystems. The level of each transcript was measured with the threshold cycle (Ct) method by using PGK1 mRNA as an endogenous control. The values were normalized to the mean of the wild-type animals in each group, which was assigned as 1 unless otherwise indicated.
Histological Analysis
Quadriceps muscles from 16-wk-old mice were snap frozen in isopentane. Sections of unfixed muscle tissue were cut at 6–8 µm in a −20°C cryostat and processed for hematoxylin and eosin or nicotinamide adenine dinucleotide staining. For immunofluorescence analysis, sections were fixed with 4% paraformaldehyde (PFA) and incubated overnight at 4°C with rabbit anti-laminin (1:80; Sigma). Digital images were captured using a Zeiss Axiovert 100M microscope and analyzed with NIS Elements software for total cross-sectional area (original magnification 10×), total myofiber number (original magnification 10×), myofiber diameter (original magnification 20×) and minimal Feret diameter (original magnification 20×). Tissue sections (8 µm thick) were fixed in 4% PFA, stained with mouse 1C2 antibody (1:20,000; Chemicon) and counterstained with Mayer hematoxylin. For muscle, >500 fibers were counted in randomly selected areas of individual mice. For motor neuron count, spinal cords from anesthetized mice were collected and postfixed for 12 h in 4% PFA. Paraffin-embedded spinal cords were serially sectioned at 6-µm steps, mounted on slides and processed for Nissl staining. Images of 10 contiguous sections, 100 µm apart (original magnification 10×), were analyzed. Motor neurons were identified as cells positive for Nissl staining, with clear nucleus and nucleolus, and a maximum diameter greater than 25 µm. Counting was performed in a blinded fashion.
Statistical Analysis
A two-way mixed-design analysis of variance (ANOVA) was done to compare the effects of genotype on hanging wire performance and body weight over time. Genotype was used as a between-subject factor, and time was used as a within-subject factor. A log-rank test was used to compare Kaplan-Meier survival between treated versus nontreated AR97Q mice. A two-tailed Student t test or, for data not normally distributed, the Mann-Whitney U test was performed to compare differences between two groups. The data were analyzed using SPSS version 18 software, and p ≤ 0.05 was considered significant.
All supplementary materials are available online at www.molmed.org .
Results
IGF-1 Administration Activates Akt and Reduces Mutant AR Aggregation in Muscle
To investigate whether systemic IGF-1 treatment is effective in SBMA mice, we used mecasermin rinfabate (rhIGF-1/IGFBP3). The circulating half-life of this compound after subcutaneous injection is longer than for IGF-1 alone, and adverse effects related to peak activity, mainly hypoglycemia, may be reduced (26). It has been used in previous clinical trials and is approved by the U.S. Food and Drug Administration for treatment of primary IGF-1 deficiency (27,28). Our study was done in transgenic mice that express the human full-length AR with 97 glutamine residues (AR97Q) (11), the same mice previously used to test the effect of transgenic overexpression of mIGF-1 on SBMA pathogenesis (22). To provide clinically relevant results, we started treatment at age 10 wks, after the onset of disease manifestations (11). A randomized cohort of male SBMA mice received either vehicle or rhIGF-1/IGFBP3 (15 mg/kg) by daily intraperitoneal injections for 6 wks. All investigators were blinded to the study agent versus vehicle until after the study was completed.
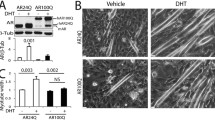
We first assessed whether treatment of SBMA mice with IGF-1 leads to activation of Akt in skeletal muscle. Akt activation was detected with a serine 473–phospho-specific antibody (29). By Western blotting analysis, we found that the IGF-1 treatment resulted in a significant increase in Akt phosphorylation at serine 473 (Figure 1A). These findings indicate that systemic treatment of SBMA mice results in activation of Akt in muscle.
IGF-1 increases phosphorylation of Akt and decreases AR aggregation in SBMA muscle. (A) Western blot analysis of Akt phosphorylation in skeletal muscle. Quantification of Akt, showing the mean with standard deviation. **p < 0.01 (n = 5). (B) Western blot analysis of AR protein levels in skeletal muscle. Quantification, showing the mean with standard deviation. *p < 0.05; **p < 0.01 (n = 5). (C) Immunohistochemical analysis using 1C2 antibody on cross-sections of AR97Q muscle shows a reduction of diffuse nuclear staining and nuclear inclusions in IGF-1 compared with vehicle-treated mice. Graphs, mean ± standard error of the mean (SEM); *p = 0.03. Scale bar 20 µm.
Expansion of polyglutamine tracts leads to accumulation of mutant protein in aggregates and inclusions (30). We have previously shown that IGF-1 treatment of SBMA cells and transgenic overexpression of IGF-1 in skeletal muscle in SBMA mice decreases the aggregation of mutant AR (21,22). Therefore, we investigated whether systemic delivery of IGF-1 has an effect on mutant AR aggregation in the SBMA mice. Here, we define aggregates as high–molecular weight oligomers soluble in radioimmunoprecipitation assay buffer after high-speed centrifugation and sonication, which can be detected as a smear in the stacking portion of SDS-polyacrylamide gels, as previously described (21,31,32). Western blot analysis showed that IGF-1 treatment reduces the accumulation of both monomeric and aggregated mutant AR in the skeletal muscle of AR97Q mice (Figure 1B). No changes were seen in the levels of endogenous mouse AR (Supplementary Figure S1). To assess whether IGF-1 affects the accumulation of mutant AR into nuclear inclusions in muscle, cross-sections of quadriceps muscle were stained with the polyglutamine-specific antibody 1C2, as previously described (22). The IGF-1 treatment reduced the number of 1C2-positive nuclei by about half (Figure 1C). Together, these results indicate that systemic treatment of SBMA mice with IGF-1 leads to Akt activation and reduction of aggregated mutant AR in muscle.
IGF-1 Attenuates Disease Manifestations in SBMA Mice
We next examined whether IGF-1 treatment ameliorates the disease phenotype in SBMA mice. Male transgenic and wildtype mice were randomly assigned to receive either vehicle or IGF-1 as described above from 10 through 20 wks of age. To assess the effect of IGF-1 on the disease manifestations, we analyzed weekly body weight (Figures 2A, B), motor function (Figures 2C, D) and survival (Figure 2E). IGF-1–treated SBMA mice had less body weight loss than vehicle-treated mice (p = 0.001; Figures 2A, B). Grip strength, assessed by a hanging wire test, significantly improved in the IGF-1–treated mice (p = 0.001; Figure 2C). Motor performance was analyzed using a Tread-Scan device at wks 10, 13 and 16. This gait analysis showed longer stride time (p = 0.01) and length (p = 0.01) and reduced rear track width (p = 0.009) and paw area (p = 0.04) in the IGF-1–treated SBMA mice compared with vehicle-treated mice at wk 16 (Figure 2D). To determine the effect of IGF-1 treatment on survival in this cohort of mice, lifespan was assessed up to a maximum of 45 wks. Mice treated with IGF-1 lived ∼3 wks longer on average than vehicle-treated mice, although this did not reach statistical significance (p = 0.09; Figure 2E), perhaps because a subset of the mice were already quite weak and close to death at 10 wks when the treatment was initiated. A post hoc analysis excluding these mice (hunched posture and sustained weight loss >5% at wk 10) showed a significant difference between the remaining animals in the two groups (p = 0.02; Supplementary Figure S2). Taken together, our results demonstrate that rhIGF-1/IGFBP3 administration ameliorates disease manifestations and extends survival in early disease stage–treated AR97Q mice.
IGF-1 attenuates weight loss and enhances motor behavior of SBMA mice. (A) Two mice from the same litter at wk 16 of age, showing the gross appearance of an IGF-1-treated (left) and vehicle-treated (right) SBMA mouse. (B, C) Body weight and hanging wire performance of SBMA mice treated with IGF-1 (n = 20) or vehicle (n = 20). Scale bars, SEM. (D) TreadScan assessment showed improved motor performance in AR97Q mice treated with IGF-1 (n = 6) compared with vehicle (n = 6). Scale bars, SEM. *p < 0.05; **p < 0.01. (E) Kaplan-Meier survival curves of mice treated with IGF-1 (n = 20) or vehicle (n = 20). p = 0.09, log-rank test.
IGF-1 Reduces Muscle Pathology in AR97Q Mice
To investigate the effects of IGF-1 treatment on SBMA muscle pathology, quadriceps muscles from 16-wk-old SBMA mice and wild-type littermates treated with vehicle or IGF-1 were collected for histopathological analyses. Muscle cross-sections of vehicle-treated AR97Q mice stained with hematoxylin and eosin (Figure 3A) and nicotinamide adenine dinucleotide (Figure 3B) showed angulated myofibers and grouped atrophic fibers as well as enlarged fibers with central nuclei. These changes are indicative of both neuropathic and myopathic changes, as previously reported (22). These signs of muscle atrophy and degeneration were markedly reduced in the rhIGF-1/IGFBP3-treated mice, indicating a protective effect of IGF-1 on muscle pathology. To further investigate the effect of IGF-1 administration on AR97Q muscle, we analyzed muscle fiber size. In the IGF-1–treated SBMA mice, the mean cross-sectional area and the minimal Feret diameter of myofibers were significantly increased compared with vehicle-treated mice (p = 0.01 and p = 0.03, respectively; Figures 3C–E). Moreover, we performed gene expression analysis of transcripts associated with muscle denervation, including myogenin and Chrna1, which we previously showed to be upregulated in both SBMA mice and patients (22). IGF-1 treatment of SBMA mice resulted in reduced expression of myogenin and Chrna1 (p = 0.003 and p = 0.0009, respectively; Supplementary Figure S3). Collectively, these results show that systemic treatment of SBMA mice with IGF-1 attenuates muscle denervation and degeneration.
IGF-1 ameliorates SBMA muscle pathology. (A) Hematoxylin and eosin staining. Arrows = fibers with central nuclei; asterisks = angulated and grouped fibers. (B) Nicotinamide adenine dinucleotide staining. Asterisks = moth-eaten fibers. (C) Histograms of myofiber cross-sectional area (CSA). (D) Muscle cross-sectional area (CSA) and (E) minimal Feret diameter were increased in SBMA mice treated with IGF-1 compared with vehicle (*p × 0.05; **p < 0.01.). Transverse sections of quadriceps of 16-wk-old SBMA mice and wild-type litter-mates are shown.
Systemic IGF-1 Treatment Has Indirect Effects on Motor Neurons in SBMA Mice
To characterize the effects of IGF-1 on the motor neurons of SBMA mice, we analyzed the spinal cord expression levels of choline acetyltransferase (ChAT), a marker for functional cholinergic neurons. ChAT levels were increased in IGF-1–treated mice compared with the vehicle-treated animals (p = 0.01; Figure 4A). Also the number of Nissl staining positive neurons with a diameter larger than 25 µm in the anterior horn of the spinal cord was slightly increased in mice treated with IGF-1, although this did not reach statistical significance (p = 0.09; Figure 4B).
hrIGF-1/IGFBP3 reduces spinal cord pathology in SBMA mice. (A) Western blot of ChAT levels in spinal cord. Quantification of ChAT level, showing the mean with standard deviation, is demonstrated (*p = 0.01). (B) Nissl-stained transverse sections of ventral spinal cords of 16-wk-old mice. Quantitative analysis showed that the number of motor neurons per section is slightly increased in AR97Q mice treated with IGF-1 compared with vehicle (p = 0.09). Graphs, mean ± SEM, n = 3. (C) Western blot of Akt phosphorylation in the spinal cord. Quantification of Akt, showing the mean with standard deviation, is demonstrated (p = 1.00). (D) Western blot of AR protein levels in spinal cord of AR97Q mice. Quantification for mutant AR and of the AR aggregates, showing the mean with standard deviation, is demonstrated (p = 0.18 and p = 0.13, respectively).
No changes in the levels of Akt phosphorylation (Figure 4C) and of monomeric and aggregated mutant AR (p = 0.18 and p = 0.13, respectively; Figure 4D) were detected in the spinal cord. Altogether these results support the idea that an increase in Akt activation in muscle with IGF-1 treatment has an indirect effect on motor neurons in the spinal cord of SBMA mice.
Discussion
Since the identification of the causative mutation, much has been learned about the molecular mechanism of SBMA through both in vivo and in vitro studies. The AR is a member of the nuclear hormone receptor superfamily (33) and resides in the cytoplasm when inactive. Upon androgen binding, the AR dissociates from heat shock proteins in the cytoplasm, translocates into the nucleus, binds DNA and activates and represses target genes. These steps are important in SBMA pathogenesis (34). In addition, the AR undergoes posttranslational modifications, which can have an important impact on the toxicity of the mutant protein. One such posttranslational modification relevant for SBMA pathogenesis is phosphorylation. We have previously shown that, in cell culture, IGF-1 induces phosphorylation of mutant AR via Akt, blocks ligand binding and reduces its toxicity (21). SBMA mice overexpressing the muscle isoform of IGF-1 have a milder phenotype (22).
IGF-1, also known as somatomedin C, is a 70–amino acid peptide homologous to proinsulin. It is released from liver after growth hormone stimulation. In addition, IGF-1 is synthesized in a number of target tissues, where it is thought to act locally in a paracrine fashion. IGF-1 forms complexes with at least six IGF-1–binding proteins, which increase its half-life, with IGFBP3 binding the largest fraction (∼75%). IGF-1 has substantial effects on skeletal muscle, promoting and regulating muscle growth and differentiation (35,36), and on motor neurons, improving sprouting, axonal growth and cell survival (37). In vivo studies have shown that IGF-1 prevents motor neuron death after nerve injury in neonatal rats (38,39) and slows motor neuron disease progression in the wobbler mouse (40).
For its combined effects on neurons and muscle, human motor neuron diseases, such as amyotrophic lateral sclerosis, present an ideal opportunity for therapeutic intervention with IGF-1. However, to date, the response of peripheral delivery of IGF-1 has been not beneficial in amyotrophic lateral sclerosis clinical trials (41–43). SBMA may be a better candidate for IGF-1 administration, because IGF-1 specifically reduces the toxicity of mutant AR via Akt phosphorylation (21,22) and because muscle, which is readily accessible to systemic IGF-1 treatment, is likely to be involved in the pathogenesis of SBMA. Muscle biopsies from SBMA patients show myogenic changes as well as neurogenic atrophy (44), muscle pathology precedes spinal cord pathology in a knock-in mouse model of SBMA (45) and muscle-specific overexpression of wild-type AR leads to an SBMA-like phenotype (46). Therefore, it is appropriate to target muscle with peripheral delivery of IGF-1 in SBMA.
In this study, we evaluated the effects of daily intraperitoneal injection of IGF-1/IGFBP3 in a mouse model of SBMA. The combination of IGF-1 and IGFBP3 mimics the biological effects of IGF-1 alone but has a more favorable pharmacokinetic profile and less adverse effects (26).
The treatment improved motor performance, attenuated weight loss, improved muscle pathology and increased survival in AR97Q mice. It also had indirect neurotrophic effects on motor neurons. Skeletal muscle is well known to be a source of signals that are retrogradely transported by motor neurons (47) and influence motor neuronal survival, growth and maintenance (48). Our results indicate that systemic delivery of IGF-1 can modify AR polyglutamine toxicity in vivo. Because IGF-1 supplementation is not known to affect male fertility, testosterone levels were not measured in our cohort of mice.
Notably, in this study, we adopted an approach relevant to the clinical setting. Treatment allocation was randomized, and investigators were kept blind throughout the study. Also, the injections were started after the onset of disease manifestations. This is especially important for a disease such as SBMA, which is generally not diagnosed until after the onset of overt weakness.
Conclusion
Our study indicates that IGF-1 administration is beneficial in SBMA mice. These results provide a preclinical basis for further examining IGF-1 in patients with SBMA.
Disclosure
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. (1991) Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 352:77–9.
La Spada AR, et al. (1992) Meiotic stability and genotype-phenotype correlation of the trinucleotide repeat in X-linked spinal and bulbar muscular atrophy. Nat. Genet. 2:301–4.
Adachi H, et al. (2005) Widespread nuclear and cytoplasmic accumulation of mutant androgen receptor in SBMA patients. Brain. 128:659–70.
Kennedy WR, Alter M, Sung JH. (1968) Progressive proximal spinal and bulbar muscular atrophy of late onset: a sex-linked recessive trait. Neurology. 18:671–80.
Rhodes LE, et al. (2009) Clinical features of spinal and bulbar muscular atrophy. Brain. 132:3242–51.
Sambataro F, Pennuto M. (2011) Cell-autonomous and non-cell-autonomous toxicity in polyglutamine diseases. Prog. Neurobiol. 97:152–72.
Bricceno K, Fischbeck KH, Burnett BG. (2012) Neurogenic and myogenic contributions to hereditary motor neuron disease. Neurodegener. Dis. 9:199–209.
Warner CL, et al. (1992) X-linked spinomuscular atrophy: a kindred with associated abnormal androgen receptor binding. Neurology. 42:2181–2184.
Sinnreich M, Klein CJ. (2004) Bulbospinal muscular atrophy: Kennedy’s disease. Arch. Neurol. 61:1324–6.
Orr HT, Zoghbi HY. (2007) Trinucleotide repeat disorders. Annu. Rev. Neurosci. 30:575–621.
Katsuno M, et al. (2002) Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal bulbar muscular atrophy. Neuron. 35:843–54.
Takeyama K, et al. (2002) Androgen-dependent neurodegeneration by polyglutamine-expanded human androgen receptor in Drosophila. Neuron. 35:855–64.
Katsuno M, et al. (2003) Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat. Med. 9:768–73.
Katsuno M, et al. (2010) Japan SBMA Interventional Trial for TAP-144-SR (JASMITT) study group: efficacy and safety of leuprorelin in patients with spinal and bulbar muscular atrophy (JASMITT study): a multicenter, randomized, double-blind, placebo-controlled trial. Lancet Neurol. 9:875–84.
Fernández-Rhodes LE, et al. (2011) Efficacy and safety of dutasteride in patients with spinal and bulbar muscular atrophy: a randomized placebo-controlled trial. Lancet Neurol. 10:140–7.
Chevalier-Larsen ES, et al. (2004) Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy. J. Neurosci. 24:4778–86.
Sopher BL, et al. (2004) Androgen receptor YAC transgenic mice recapitulate SBMA motor neuronopathy and implicate VEGF164 in the motor neuron degeneration. Neuron. 41:687–99.
Emamian ES, et al. (2003) Serine 776 of ataxin-1 is critical for polyglutamine-induced disease in SCA1 transgenic mice. Neuron. 38:375–87.
Chen HK, et al. (2003) Interaction of Akt-phosphorylated ataxin-1 with 14–3–3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell. 113:457–68.
Gu X, et al. (2009) Serines 13 and 16 are critical determinants of full-length human mutant huntingtin induced disease pathogenesis in HD mice. Neuron. 64:828–40.
Palazzolo I, et al. (2007) Akt blocks ligand binding and protects against expanded polyglutamine androgen receptor toxicity. Hum. Mol. Genet. 16:1593–603.
Palazzolo I, et al. (2009) Overexpression of IGF-1 in muscle attenuates disease in a mouse model of spinal and bulbar muscular atrophy. Neuron. 63:316–28.
Committee for the Update of the Guide for the Care and use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council of the National Academies. (2011) Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press. [cited 2012 Oct 29]. Available from: https://doi.org/oacu.od.nih.gov/regs/
Crawley JN. (2007) What’s Wrong with My Mouse? Hoboken, NJ: John Wiley & Sons, p. 72–73.
Pennuto M, et al. (2008) Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron. 57:393–405.
Camacho-Hübner C, et al. (2006) Pharmacokinetic studies of recombinant human insulin-like growth factor 1 (rhIGF-1)/rhIGF-binding protine-3 complex administered to patients with growth hormone insensitivity syndrome. J. Clin. Endocrinol. Metab. 91:1246–53.
Debroy MA, et al. (1999) Anabolic effects of insulin-like growth factor in combination with insulin-like growth factor binding protein-3 in severely burned adults. J. Trauma. 47:904–10.
Clemmons DR, Sleevi M, Allan G, Sommer A. (2007) Effects of combined recombinant insulin-like growth factor (IGF)-I and IGF binding protein-3 in type 2 diabetic patients on glycemic control and distribution of IGF-I and IGF-II among serum binding protein complexes. J. Clin. Endocrinol. Metab. 92:2652–8.
Jacinto E, et al. (2006) SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 127:125–37.
Li M, et al. (1998) Nuclear inclusions of the androgen receptor protein in spinal and bulbar muscular atrophy. Ann. Neurol. 44:249–54.
Li M, Chevalier-Larsen ES, Merry DE, Diamond MI. (2007) Soluble androgen receptor oligomers underlie pathology in a mouse model of spinobulbar muscular atrophy. J. Biol. Chem. 282:3157–64.
Rusmini P, et al. (2007) Aggregation and proteasome: the case of elongated polyglutamine aggregation in spinal and bulbar muscular atrophy. Neurobiol. Aging. 28:1099–111.
Parodi S, Pennuto M. (2011) Neurotoxic effects of androgens in spinal and bulbar muscular atrophy. Front Neuroendocrinol. 32:416–25.
Nedelsky NB, et al. (2010) Native function of the androgen receptor are essential to pathogenesis in a Drosophila model of spinobulbar muscular atrophy. Neuron. 67:936–52.
Duan C, Ren H, Gao S. (2010) Insulin-like growth factors (IGFs), IGF-receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen. Comp. Endocrinol. 167:344–51.
Sandri M, et al. (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 117:399–412.
Caroni P, Grandes P. (1990) Nerve sprouting in innervated adult skeletal muscle induced to exposure to elevated levels of insulin-like growth factors. J. Cell. Biol. 110:1307–17.
Li L, Oppenheim RW, Lei M, Houenou LJ. (1994) Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J. Neurobiol. 25:759–66.
Yuan Q, et al. (2000) Effects of neurotrophic factors on motoneuron survival following axonal injury in newborn rats. Neuroreport. 11:2237–41.
Gorio A, Lesma E, Madaschi L, Di Giulio AM. (2002) Co-administration of IGF-I and glycosaminoglycans greatly delays motor neurone disease and affects IGF-I expression in the wobbler mouse: a long-term study. J. Neurochem. 81:194–202.
Lai EC, et al. (1997) Effect of recombinant human insulin-like growth factor-I on progression of ALS: a placebo-controlled study: the North America ALS/IGF-I Study Group. Neurology. 49:1621–30.
Borasio GD, et al. (1998) A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. European ALS/IGF-I Study Group. Neurology. 51:583–6.
Sorenson EJ, et al. (2008) Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology. 71:1770–5.
Sorarù G, et al. (2008) Spinal and bulbar muscular atrophy: skeletal muscle pathology in male patients and heterozygous females. J. Neurol. Sci. 264:100–5.
Yu Z, et al. (2006) Androgen-dependent pathology demonstrates myopathic contribution to the Kennedy disease phenotype in a mouse knock-in model. J. Clin. Invest. 116:2663–72.
Monks DA, et al. (2007) Overexpression of wildtype androgen receptor in muscle recapitulates polyglutamine disease. Proc. Natl. Acad. Sci. U. S. A. 104:18259–64.
DiStefano PS, et al. (1992) The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 8:983–93.
Funakoshi H, et al. (1995) Muscle-derived neu-rotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 268:1495–9.
Acknowledgments
We would like to thank Hiroaki Adachi for helping with the 1C2 immunostaining, Sweta Girgenrath for discussion about dose selection and Nico P Dantuma for support. LC Bott was part of the National Institutes of Health–Karolinska Graduate Partnership Program in Neuroscience. Insmed provided the study agent and placebo.
This work was supported by NINDS intramural funds. The work was also supported by grants from Telethon-Italy (GGP10037), the Kennedy’s Disease Association and the Muscular Dystrophy Association (196646) to M Pennuto.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit (https://doi.org/creativecommons.org/licenses/by-nc-nd/4.0/)
About this article
Cite this article
Rinaldi, C., Bott, L.C., Chen, Kl. et al. Insulinlike Growth Factor (IGF)-1 Administration Ameliorates Disease Manifestations in a Mouse Model of Spinal and Bulbar Muscular Atrophy. Mol Med 18, 1261–1268 (2012). https://doi.org/10.2119/molmed.2012.00271
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2012.00271