Abstract
Rheumatoid arthritis (RA) patients are characterized by increased arterial stiffness, an independent predictor of cardiovascular risk. It has been suggested that osteopontin (OPN), a cytokine involved in RA pathogenesis, might have vascular effects. To study a possible relationship between OPN and arterial stiffness, aortic pulse wave velocity (PWV) was measured by tonometry in 69 patients (41 with RA, 28 with systemic sclerosis (SSc)) and 18 healthy controls. Plasma OPN levels, oxidative stress markers, and endothelin 1 (ET-1) were assessed. OPN levels were significantly (P< 0.05) higher in RA (median 9.93, range 4.36–47.80 ng/mL) than in SSc (4.3, 2.1–19.7 ng/mL) or controls (5.2, 4.1–9.4 ng/mL). In RA patients, log-OPN was related to log-C-reactive protein (log-CRP) (r= 0.30, P< 0.05), age (r= 0.38, P< 0.01), Health Assessment Questionnaire (HAQ) (r= 0.58, P< 0.0001), and inversely related to total cholesterol (r= −0.33, P< 0.05) and apolipoprotein A (apoA) (r= −0.58, P< 0.001), but not to oxidative stress markers and ET-1. PWV was similar in RA (median 8.1, range 4.7–16.4 m/s) and SSc (median 8.7, range 7.1–13.1 m/s), but significantly greater (P < 0.01) than controls (median 7.5, range 4.1–10.4 m/s). Aortic PWV was related to log-OPN (r= 0.40, P < 0.01) only in RA patients. It also was related to age (r= 0.34, P< 0.05), mean blood pressure (r= 0.44, P< 0.001), and HAQ (r = 0.48, P< 0.001). In multiple regression analysis (r2 = 0.36), including confounders, log-OPN remained a significant predictor (P < 0.05) of PWV in RA. Elevated plasma OPN levels are associated with increased arterial stiffness in RA patients, suggesting that this protein might represent a bridge protein between inflammation and the consequent joint damage and cardiovascular risk in RA patients.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic progressive inflammatory disease, characterized by synovial inflammation and hyperplasia, leading to progressive cartilage and bone destruction (1). Patients with RA have a shortened life span and cardiovascular diseases caused by accelerated atherosclerosis (2) are the most common cause of mortality in these patients (3–7).
Recent studies have shown a possible role of RA as an independent risk factor for atherosclerosis, since traditional cardiovascular disease risk factors could be insufficient to explain the high incidence of cardiovascular events (8,9). In particular, RA with extra-joint involvement, associated with high inflammation, is associated with greater cardiovascular mortality, suggesting a direct role of RA in the pathogenesis of cardiovascular damage (10).
Increased arterial stiffness (a function of aging and characterized by a reduction in elastin and an increase in collagen) can be measured as pulse wave velocity (PWV) (12). PWV is a vascular parameter of important clinical significance since it has been demonstrated to be an independent predictor of cardiovascular events both in high-risk patients and in the general population (12). Increased arterial stiffness was demonstrated in RA patients at the abdominal aorta (13) and carotid site (14), and as elevated aortic PWV (15). The latter alteration was related to C-reactive protein (CRP) levels, and was reversible after anti-TNF therapy (15,16); but it also was associated with risk factors such as age, blood pressure, and abdominal obesity (17). Interestingly, aortic PWV was related to paraarticular trabecular bone loss at the ultradistal radius (18).
Physiologically, osteopontin (OPN) is a potent inhibitor of mineralization; it prevents ectopic calcium deposits, and is a potent inducible inhibitor of vascular calcification (19). On the other hand, this cytokine is expressed in chronic inflammatory and autoimmune diseases (20–25) with proinflammatory functions, and it has been suggested as a potential mediator of the promotion of joint destruction in RA (26). Enhanced levels of OPN mRNA and protein were found in synovial tissue from RA patients (27). Moreover, OPN deficiency has been shown to protect joints against destruction in arthritis-induced mice—suppressing articular destruction, chondrocyte apoptosis, and synovial angiogenesis (28), suggesting a direct role in bone readsorption beyond its effects on inflammation (29). It also has been hypothesized that OPN has a role in atherosclerosis, since elevated levels of OPN are associated with both the extent of cardiovascular disease, independently of traditional risk factors (30), and with restenosis (31).
Based on reported literature, the aim of the present study was to investigate the possible relationship between plasma OPN levels with an established vascular parameter of risk such as aortic PWV in RA patients. The interaction with disease activity and severity, inflammatory and oxidative stress markers, and common atherosclerotic risk factors also were evaluated.
Plasma OPN levels and aortic PWV will be evaluated both in a control group and in a group of patients affected by systemic sclerosis (SSc). The SSc group serves as a non-case group characterized by inflammation, ectopic calcification, and vasculites, but without the articular involvement typical of RA patients.
Materials and Methods
Patients and OPN Measurements
Forty-one RA patients (30 Female [F], 11 Male [M]; mean age ± standard deviation [SD]: 58.8 ± 10.3 years) were recruited who fulfilled the American College of Rheumatology (ACR, formerly, the American Rheumatism Association) 1987 revised criteria for RA (32). Duration of disease was 11 ± 9 years (mean ± SD). A non-case group (25 F, 3 M; 60.4 ± 9.4 years) consisting of 28 SSc patients, and 18 healthy volunteers (controls) (13 F, 5 M; 54.9 ± 7.8 years) also were recruited.
Patients were recruited at the Division of Rheumatology (University of Pisa), healthy controls were either friends or neighbors of patients.
Exclusion criteria for patients and controls were: past coronary angina, previous myocardium infarction, cerebral ischemic stroke, or hypertensive subjects with values of the arterial pressure ≥ 140/90 mmHg or currently in antihypertensive drug treatment. Patients with statin and nonsteroidal antiinflammatory treatment also were excluded.
Patients (RA and SSc) and controls were examined to evaluate the common cardiovascular (CV) risk factors for coronary artery disease such as positive family history, smoking, diabetes mellitus, or dislipidemia.
RA patient assessment included a standard assessment of disease activity and severity, including the following parameters: tender and swollen joint count (TJC and SJC, respectively), 44 joint count for swelling (SW44), general health status (GH), Disease Activity Score (DAS28), erythrocyte sedimentation rate (ESR), CRP for the evaluation of disease activity, modified Health Assessment Questionnaire (HAQ) score (32), joint deformities, extraarticular features, and erosions.
The protocol was approved by the Ethical Committee of the University of Pisa, and written informed consent was obtained from each participant.
Experimental Procedures
Determination of OPN. For plasma OPN measurement, blood samples (3 mL) of RA and SSc patients, and healthy controls were collected in EDTA-containing tubes early in the morning. Samples were centrifuged (400g, 15 min, at 4° C) to remove cells and debris, and stored at −80° C until used. Human Osteopontin TiterZyme Enzyme Immunometric Assay (EIA) kit (Gentaur Europe, Brussels, Belgium) was used to perform the quantitative determination of plasmatic OPN. The kit utilized a monoclonal antibody to human OPN.
Evaluation of inflammation markers, stress oxidative factors, and common atherosclerotic risk. All these parameters were evaluated in RA and SSc patients, and controls.
The unspecific markers of inflammation ESR and CRP were analyzed. Rheumatoid factor (RF) was determined by nephelometry.
Atherosclerotic risk was evaluated by means of the quantitative analysis of total cholesterol, triglycerides, apolipoproteins apoA1 and apoB, and homocysteine.
Oxidative stress factors also were analyzed: lipid peroxides, malondialdehyde (MDA), the ferric reducing ability of plasma (FRAP), and endothelin 1 (ET-1). A sensitive enzyme immunoassay (EIA) for human endothelin (1–21) was used (Biomedica Gruppe, Wien, Austria). Lipid peroxides were analyzed by thiobarbituric acid reaction (33). FRAP was evaluated according to Benzie et al. (34). MDA was evaluated by means of the colorimetric assay for lipid peroxidation (OxisResearch, Biotech LP586, OXIS Health Products Inc., Portland, OR, USA).
Vascular function. Blood pressure (BP) was measured three times at 3 min intervals by an automatic device (OMRON-950 CP, OMRON Healthcare Europe, Hoofddorp, The Netherlands) at the dominant arm and calculated as the mean value of the last two measurements. All measurements were performed after an overnight fast, with the subjects in supine position in a quiet, air-conditioned room (22°–24° C).
Arterial tonometry was performed by one trained operator (35) according to the international recommendations (12). A hand held probe was placed on the artery and 10–15 subsequent images were recorded. Aortic PWV was assessed (SphygmoCor, AtCor Medical, Sydney, Australia) by recording waveforms at the femoral and carotid site sequentially as distance-to-time ratio. Surface distance between the two recording sites was measured and a simultaneously recorded ECG was used as a reference frame to calculate wave transit time. Coefficient of variation for PWV was 13% (35).
Statistical Analysis
Because of skewed distribution of OPN and aortic PWV, these variables were logarithmically transformed and expressed as median and range.
Data were analyzed using the non-parametric Mann-Whitney U test, and multiple regression. Spearman’s correlation test was used for correlation analysis. A P value < 0.05 was taken as the level of statistical significance. All statistical analyses were performed using SPSS statistical software, Version 9.
Results
Clinical characteristics of RA and SSc patients and controls are shown in Table 1. The three groups were comparable for age, gender distribution, blood pressure, and metabolic profile. CRP levels were greater in RA patients and similar in SSc patients and controls. Disease activity variables of RA patients are shown in Table 2.
OPN levels were significantly higher (P < 0.05) in RA patients (median: 9.93, range 4.36–47.80 ng/mL) as compared with the other two groups, in which OPN levels were not different (SSc patients: 4.3, range: 2.1–19.7 ng/mL, healthy controls: 5.2; 4.1–9.4 ng/mL) (Figure 1).
Box plots show plasma OPN levels and carotid to femoral PWV (median and interquartile range) in RA, SSc, and controls. OPN: RA versus SSc: P< 0.05, RA versus controls: P< 0.05, SSc versus controls: P= n.s. PWV: RA versus SSc: P= n.s., RA versus controls: P< 0.01, SSc versus controls: P< 0.01. n.s., nonsignificant.
Patients with RA exhibited significantly increased (P < 0.01) aortic PWV (median 8.1, range 4.7–16.4 m/s) as compared with controls (median 7.5, range 4.1–10.4 m/s) (see Figure 1). Aortic PWV also was significantly greater (P < 0.01) in SSc patients (median 8.7, range 7.1–13.1 m/s) than controls, but not different from RA patients (see Figure 1).
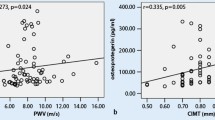
In RA patients, logistic regression showed that OPN levels above the median value were associated with a significantly increased risk for having aortic PWV above the median value of 7.6 m/s (odds ratio of 4.3, 95% confidence intervals: 1.09–17.2). Moreover, only in RA patients, log-OPN was related to log-aortic PWV (r = 0.40, P < 0.01) (Figure 2). In this group, log-OPN was related to log-CRP (r = 0.30, P < 0.05), age (r = 0.38, P < 0.01), and inversely related to total cholesterol (r = −0.33, P < 0.05) and apoA (r = −0.58, P < 0.001), but not to mean BP. On the other hand, log-aortic PWV also was related to age (r = 0.38, P < 0.01), and mean BP (r = 0.53, P < 0.0001), but not to cholesterol levels or log-CRP.
No correlations were found between log-OPN and oxidative stress markers, the other common atherosclerotic risk (except cholesterol), and ET-1.
Multiple regression analysis (r2 = 0.49), including possible confounders such as age, mean BP, cholesterol levels, and log-CRP, showed that log-OPN remained a significant predictor (P < 0.05) of log-aortic PWV.
Log-OPN was related to HAQ (r = 0.58, P < 0.0001) but not SW44 and DAS28. Aortic PWV was related to HAQ (r = 0.44, P < 0.01), while no correlations were found with SW44 and DAS28.
Discussion
OPN is rapidly emerging as a major player in both physiological and pathological processes throughout the body, as is evident from data that has emerged from studies conducted over the past few years. Literature showed that OPN is involved in the evolution and progression of multiple systemic diseases (36); it has been linked to the aethiopathogenesis of breast cancer (37), osteoporosis, multiple sclerosis (21,38), inflammatory bowel diseases (22), psoriasis (20,25), and lupus erythematosus systemicus (24,39). There is evidence that OPN also may be implicated in RA pathogenesis (23,40).
Our aim was to study a possible relationship between OPN and arterial stiffness, measured by aortic PWV, in RA patients. PWV is considered the gold standard for assessing arterial stiffness, and is an independent predictor of CV events in high-risk patients and in the general population (12).
Results showed that RA patients exhibited higher plasmatic OPN levels (approximately two times) compared with the healthy subjects and to the non-case SSc patients, suggesting a peculiar role for this protein in RA pathogenesis. This is supported by the associations found in RA patients between log-OPN and the clinical parameters HAQ, and log-OPN versus log-PWV.
RA patients exhibited higher aortic PWV compared with controls, according to published literature (15), and similar PWV to SSc patients, but the novelty is the association between log-OPN and log-PWV, which showed significant results (P < 0.01) only in RA patients.
Considering the known properties of OPN as a regulator of inflammation and bone destruction in RA (26,27,41), we suggest that OPN might be a point of contact between inflammation and the consequent articular damage and atherosclerosis in RA. The observed lower OPN levels of non-case and healthy donors led us to speculate that this inflammatory and plaque destabilizing protein is peculiar in RA pathogenesis. This hypothesis is supported by the fact that patients with SSc, who are characterized by inflammation, ectopic calcification, and vasculites, but who don’t have articular involvement, have similar OPN levels as compared with healthy subjects; moreover we have found that OPN levels correlated with PWV only in RA patients, even if PWV levels were similar in RA and SSc.
In multiple regression analyses, including the possible confounders such as age, mean BP, cholesterol levels, and log-CRP, log-OPN remained a significant predictor of log-aortic PWV in RA patients.
Although the RA patients studied were free of overt CV disease, 18 patients (44%) had high levels of total cholesterol. A negative correlation was observed between OPN levels and total cholesterol, and OPN levels and apoA. Also Take-moto et al. (41) observed, as we did, a significant negative correlation between the plasma OPN level and serum total cholesterol concentration.
Literature suggests a role for OPN in RA pathogenesis. Enhanced levels of OPN mRNA and proteins were found in synovial tissue from RA patients (26), and OPN deficiency has been shown to protect joints against destruction in arthritis-induced mice (28).
Recently, a candidate treatment to suppress OPN expression, based on a novel murine anti-OPN, 23C3, has been developed, and shows great potential in the treatment of RA. In fact, administration of 23C3 to mice with collagen-induced arthritis (CIA) not only strongly suppresses the development of CIA, but also decreases the severity of the existing arthritis (42).
Moreover, an OPN promoter polymorphism at −66G and −443C recently has been identified to be associated with carotid artery intima media thickness, which reflects generalized atherosclerosis, and is predictive of future vascular events (43,44).
In conclusion, our results suggest that patients affected by RA, free from CV risk factors and overt CV disease, showed high levels of plasmatic OPN, and that OPN levels correlated with a clinical severity index, and with a marker of atherosclerosis and CV disease such as PWV.
Moreover, in SSc patients (who are characterized by ectopic calcification and inflammation, but do not have the bone erosion seen in RA patients) the levels of OPN are much lower than those in RA patients. In addition, SSc patients do not have OPN related to PWV.
We hypothesize an active role of circulating OPN in RA pathogenesis. OPN might also act as an unfavorable CV risk factor in patients with RA by perpetuating the inflammatory state that promotes CV disease along with classical risk factors in RA. Our results suggest this protein might represent a bridge protein between inflammation, the consequent articular damage, atherosclerosis, and CV risk in RA patients.
Disclosure
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
Bazzichi L, et al. (2005) Quality of life in rheumatoid arthritis: impact of disability and lifetime depressive spectrum symptomatology. Clin. Exp. Rheumatol. 23:783–8.
Riise T, Jacobsen BK, Gran JT, Haga HJ, Arnesen E. (2001) Total mortality is increased in rheumatoid arthritis. A 17-year prospective study. Clin. Rheumatol. 20:123–7.
Lévy L, Fautrel B, Barnetche T, Schaeverbeke T. (2008) Incidence and risk of fatal myocardial infarction and stroke events in rheumatoid arthritis patients. A systematic review of the literature. Clin. Exp. Rheumatol. 26:673–679.
Gazi IF, Boumpas DT, Mikhailidis DP, Ganotakis ES. (2007) Clustering of cardiovascular risk factors in rheumatoid arthritis: the rationale for using statins. Clin. Exp. Rheumatol. 25:102–111.
Mutru O, Laakso M, Isomäki H, Koota K. (1989) Cardiovascular mortality in patients with rheumatoid arthritis. Cardiology. 76:71–7.
Wolfe F, et al. (1994) The mortality of rheumatoid arthritis. Arthritis Rheum. 37:481–94.
Libby P. (2008) Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am. J. Med. 121(10 Suppl 1):S21–31.
del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. (2001) High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 44:2737–45.
Goodson N. (2002) Coronary artery disease and rheumatoid arthritis. Curr. Opin. Rheumatol. 14:115–20.
Van Doornum S, McColl G, Wicks IP. (2002) Accelerated atherosclerosis: an extraarticular feature of rheumatoid arthritis? Arthritis Rheum. 46:862–73.
Lakatta EG. (2003) Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 107:490–7.
Laurent S, et al. (2006) Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J. 27:2588–605.
Turesson C, et al. (2005) Increased stiffness of the abdominal aorta in women with rheumatoid arthritis. Rheumatology (Oxford). 44:896–901.
Roman MJ, et al. (2005) Arterial stiffness in chronic inflammatory diseases. Hypertension. 46:194–9.
Maki-Petaja KM, et al. (2006) Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation. 114:1185–92.
Cypiene A, et al. (2007) Non-invasive assessment of arterial stiffness indices by applanation tonometry and pulse wave analysis in patients with rheumatoid arthritis treated with TNF-alpha blocker remicade (infliximab). Proc. West Pharmacol. Soc. 50:119–22.
Tokita A, et al. (2009) Carotid arterial elasticity is a sensitive atherosclerosis value reflecting visceral fat accumulation in obese subjects. Atherosclerosis. 2009, Feb 20 [Epub ahead of print].
Tanaka K, et al. (2006) Paraarticular trabecular bone loss at the ultradistal radius and increased arterial stiffening in postmenopausal patients with rheumatoid arthritis. J Rheumatol. 33:652–8.
Scatena M, Liaw L, Giachelli CM. (2007) Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler. Thromb. Vasc. Biol. 27:2302–9.
Chen YJ, et al. (2009) Elevated plasma osteopontin level is associated with occurrence of psoriasis and is an unfavorable cardiovascular risk factor in patients with psoriasis. J. Am. Acad. Dermatol. 60:225–30.
Altintaş A, Saruhan-Direskeneli G, Benbir G, Demir M, Purisa S. (2009) The role of osteopontin: a shared pathway in the pathogenesis of multiple sclerosis and osteoporosis? J. Neurol. Sci. 276:41–4.
Mishima R, et al. (2007) High plasma osteopontin levels in patients with inflammatory bowel disease. J. Clin. Gastroenterol. 41:167–72.
Hasegawa M, et al. (2009) Thrombin-cleaved osteopontin in synovial fluid of subjects with rheumatoid arthritis. J. Rheumatol. 36:240–5.
Xu AP, et al. (2007) Osteopontin gene polymorphism in association with systemic lupus erythematosus in Chinese patients. Chin. Med. J. (Engl). 120:2124–8.
Buommino E, et al. (2009) Osteopontin: a new emerging role in psoriasis. Arch. Dermatol. Res. 301:397–404.
Petrow PK, et al. (2002) Characterization of the cell type-specificity of collagenase 3 mRNA expression in comparison with membrane type 1 matrix metalloproteinase and gelatinase A in the synovial membrane in rheumatoid arthritis. Ann. Rheum. Dis. 61:391–7.
Ohshima S, et al. (2002) Expression of osteopontin at sites of bone erosion in a murine experimental arthritis model of collagen-induced arthritis: possible involvement of osteopontin in bone destruction in arthritis. Arthritis Rheum. 46:1094–101.
Yumoto K, et al. (2002) Osteopontin deficiency protects joints against destruction in anti-type II collagen antibody-induced arthritis in mice. Proc. Natl. Acad. Sci. U. S. A. 99:4556–61.
Jacobs JP, et al. (2004) Lack of requirement of osteopontin for inflammation, bone erosion, and cartilage damage in the K/BxN model of autoantibody-mediated arthritis. Arthritis Rheum. 50:2685–94.
Ohmori R, et al. (2003) Plasma osteopontin levels are associated with the presence and extent of coronary artery disease. Atherosclerosis. 170:333–7.
Kato R, et al. (2006) High plasma levels of osteopontin in patients with restenosis after percutaneous coronary intervention. Arterioscler. Thromb. Vasc. Biol. 26:e1–2.
Pincus T, Summey JA, Soraci SA Jr, Wallston KA, Hummon NP (1983) Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 26:1346–53.
Wolff S. Ferrous ione oxidation in presence of ferric ion indicator xylenol orange for measurement for hydroperoxydes. Methods Enzymol. 1994;233:182–189.
Benzie IF, Strain JJ. (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 299:15–27
Plantinga Y, et al. (2007) Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am. J. Hypertens. 20:392–7.
Kapoor S. (2008) Osteopontin: a major player in the pathogenesis of endocrine and systemic diseases. Endocr. J. 55:785.
Mirza M, et al. (2008) Osteopontin-c is a selective marker of breast cancer. Int. J. Cancer. 122:889–97.
Chiocchetti A, et al. (2005) Osteopontin gene haplotypes correlate with multiple sclerosis development and progression. J. Neuroimmunol. 163:172–8.
Wong CK, Lit LC, Tam LS, Li EK, Lam CW. (2005) Elevation of plasma osteopontin concentration is correlated with disease activity in patients with systemic lupus erythematosus. Rheumatology (Oxford). 44:602–6.
Xu G, et al. (2005) Role of osteopontin in amplification and perpetuation of rheumatoid synovitis. J. Clin. Invest. 115:1060–7.
Takemoto M, et al. (1999) Effects of aging and hyperlipidemia on plasma osteopontin level. Nippon Ronen Igakkai Zasshi. 36:799–802.
Du J, et al. (2008) Molecular basis of recognition of human osteopontin by 23C3, a potential therapeutic antibody for treatment of rheumatoid arthritis. J. Mol. Biol. 382:835–42.
de las Fuentes L, et al. (2008) Osteopontin promoter polymorphism is associated with increased carotid intima-media thickness. J. Am. Soc. Echocardiogr. 21:954–60.
Brenner D, et al. (2006) Cytokine polymorphisms associated with carotid intima-media thickness in stroke patients. Stroke. 37:1691–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bazzichi, L., Ghiadoni, L., Rossi, A. et al. Osteopontin Is Associated with Increased Arterial Stiffness in Rheumatoid Arthritis. Mol Med 15, 402–406 (2009). https://doi.org/10.2119/molmed.2009.00052
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2009.00052